Major Histocompatibility Complex (MHC)
What is MHC?
The major histocompatibility complex (MHC) is comprised of cell membrane proteins that deliver short peptides to the cell surface, presenting these peptide antigens to circulating immune surveillance cells. These peptides are mostly self-peptides however during times of infection these can also be antigens from pathogens that, upon recognition and subsequent binding by the immune cells, specifically T cells, result in the generation of an immune response.

An Overview of T Cell Receptors Mini-review
PDF Download Available

The T Cell Marker, CD3 Antigen and Antibodies Mini-review
PDF Download Available
The MHC-peptide complex on the cell membrane interacts with the T cell receptor (TCR) with the MHC binding to the TCR and its CD3/CD4 or CD8 co-receptors, while the antigenic epitope is bound by the variable Ig-like domain of the TCR. These interactions stimulate T cell activation. Read our mini-reviews for more detailed information on the TCR and CD3, the major T cell co-receptor.
The MHC gene expresses three types of molecule. The class III molecules are not involved in antigen presentation; instead they play a role in inflammation. These molecules include complement components such as C2 and C4, tumor necrosis factor - α and heat shock proteins. There are two main types of MHC that present antigens; MHC class I and MHC class II.
Due to the requirement of MHC interactions with TCR and its co-receptors in the appropriate activation of T cells, they also play a critical role in thymic T cell selection. The first stage in thymic T cell selection, positive selection, requires the T cells to bind to MHC Class I or MHC Class II molecules with an appropriate affinity to initiate the stimulation of “survival signals” within the T cell. The second stage of T cell selection, negative selection, removes auto-reactive T cells and, therefore prevents autoimmunity. During negative selection the majority of T cells that bind too strongly to self-antigens receive apoptotic signals which result in cell death. T cells with low affinity for self survive to become effector T cells, while those with an intermediate affinity for self may go on to become regulatory T cells. Only 2% of the thymocytes populating the thymus reach maturity and exit the thymus to circulate as immature T cells. In the periphery when self-antigen is presented to T cells, T cell activation should not take place. Autoimmune disease occurs when control of auto-recognition fails, and results in T cells being unable to tolerate self.
In humans the MHC is also known as human leukocyte antigen (HLA) complex and in the mouse it is known as the H-2 complex.
MHC Class I
Location: Present in all nucleated cells. Initially present in the endoplasmic reticulum, for peptide loading, the MHC I molecules are externalized via the Golgi apparatus and secretory vesicles which fuse with the cell membrane.
Structure: Two chains; α chain composed of three domains (α3 is transmembrane, α1 and α2 domains form the peptide binding groove) and a β2 microglobulin. This complex is stabilized by chaperone proteins; calreticulum, Erp57, protein disulphide isomerase and tapasin, until peptide recognition and binding by a T cell.
Peptides: Mostly intracellular, generated by proteasomal (26S, thymic-specific and immuno proteasomes) degradation of proteins which are subsequently transferred to the MHC complex by transporter associated with antigen processing (TAP) molecules. The presentation of intracellular peptides means that this class of MHC molecule is critical in facilitating an immune response to intracellular pathogens, such as viruses.
Peptide presentation: To cytotoxic CD8+ T cells.
Result of T cell binding: Instigation of programmed cell death by apoptosis of the MHC expressing cell.
In humans: The HLA is comprised of three molecules; HLA-A, HLA-B and HLA-C.
MHC Class II
Location: Usually only on professional antigen presenting cells (APCs): macrophages, B cells, dendritic cells (DCs). Initially present in the endoplasmic reticulum, where the MHC II complex is stabilized by the invariant chain, which also prevents it binding cellular peptides. The MHC II is then externalized via the Golgi apparatus, where the invariant chain is partially degraded to a peptide which is held in the binding groove. This invariant chain peptide is exchanged for a foreign antigen before presentation at the cell surface; involving the chaperone HLA-DM in the majority of APC, while B cells specifically require HLA-DO.
Structure: Two chains; α and β chains; both having two domains (with the α2 and β2 domains being transmembrane and the α1 and β1 domains forming the peptide binding groove).
Peptides: Mostly extracellular, generated by endocytic pathway protein degradation. The peptide is taken up by the APCs; process of phagocytosis into phagosomes by macrophages and DCs, and by endocytosis into endosomes by B cells. After antigen processing an epitope is presented in complex with MHC II on the cell surface. This mechanism therefore facilitates the generation of an immune response to external pathogens, such as fungi and bacteria.
Peptide presentation: To helper CD4+ T cells.
Result of T cell binding: Depending upon the macro environment, which includes co-stimulation molecule expression and cytokines secretion by the APCs, the helper T cell is stimulated into either a memory T cell or effector T cells. The effector T cell can be: T helper (Th) type 1, Th type 2, Th type 17 or regulatory T cell.
In humans: The HLA is comprised of six isotypes: HLA-DPA1, HLA-DPB1, HLA-DQA1, HLA-DQB1, HLA-DRA and HLA-DRB1.
Human Markers
The genes for the HLAs are highly polymorphic, with each individual having their own specific HLA allele set. This gives rise to a diversity of antigen presentation, thereby having differing susceptibility to infection and auto immunity.
In addition to the alleles known to make up MHC I and MHC II, there are a number which are considered pseudogene molecules or their role is not yet known. As there are many different alleles this results in a numbering system such as HLA-B27. Table 1 shows a number of the different markers available to detect the various molecules of the HLA.
Table 1. Human HLA markers and antibodies.
HLA |
MHC Class |
Antibody |
Clone |
|---|---|---|---|
|
HLA A |
|
|
|
|
HLA A |
MHC I |
Polyclonal |
|
|
HLA A2 |
MHC I |
BB7.2 |
|
|
HLA ABC |
MHC I |
YTH862.2, W6/32 |
|
|
HLA B |
|
|
|
|
HLA B27 |
MHC I |
HLA-ABC-m3 |
|
|
HLA B7 |
MHC I |
BB7.1 |
|
|
HLA D |
|
|
|
|
HLA DR |
MHC II |
HL-3, YE2/36-HLK, YD1/63.4.10 |
|
|
HLA DP DQ DR |
MHC II |
Bu26, WR18 |
|
|
HLA DQ |
MHC II |
PrecisionAb™ Polyclonal |
|
|
HLA E and G |
|
|
|
|
HLA E |
|
MEM-E/02 |
|
|
HLA G |
|
MEM-G/1, MEM-G/9 |
Featured Key Marker HLA ABC (MHC Class I)
There are three major MHC class I proteins encoded by the HLA which are HLA A, HLA B and HLA C. These proteins are found on the surface of almost all nucleated cells. Mouse Anti-Human HLA ABC Antibody, clone W6/32, recognizes an antigenic determinant shared among products of the HLA A, B and C loci. Clone W6/32 recognizes a conformational epitope, reacting with HLA class I α3 and α2 domains. This human HLA ABC specific antibody has been used in conjunction with the B cell marker, CD19, in flow cytometry (Figure 1), to show, as you would expect, MHC Class I expression in all B cells.
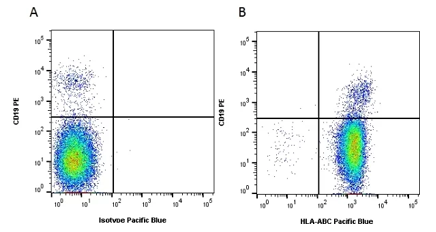
Fig.1. Flow cytometry analysis of Human HLA Marker HLA ABC. A, RPE conjugated Mouse Anti-Human CD19 (MCA1940PE) and Pacific Blue conjugated Mouse IgG2a Isotype Control (MCA929PB). B, RPE conjugated Mouse Anti-Human CD19 (MCA1940PE) and Pacific Blue conjugated Mouse Anti-Human HLA ABC (MCA81PB). All experiments performed on red cell lysed human blood gated on lymphocytes. Data acquired on the ZE5™ Cell Analyzer.
Featured Key Marker HLA DP DQ DR (MHC Class II)
There are three major MHC class II proteins encoded by the HLA; HLA-DP, HLA-DQ and HLA-DR. Found on APCs such as macrophages and DCs. Mouse Anti-Human HLA DP DQ DR Antibody, clone WR18, recognizes a monomorphic determinant common to HLA DP, HLA DQ and HLA DR. This human HLA DP DQ DR specific antibody has been used in conjunction with the DC cell marker, CD11c, in flow cytometry (Figure 2), to show MHC Class II expression in DC’s.
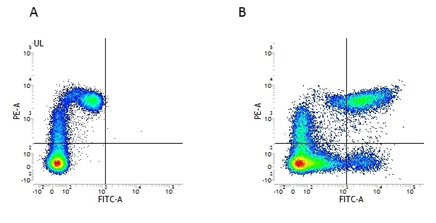
Fig.2. Flow cytometry analysis of Human HLA Marker HLA DP DQ DR. A, RPE conjugated Mouse Anti-Human CD11c (MCA2087PE) and FITC conjugated Mouse IgG2a Isotype Control (MCA929F). B, RPE conjugated Mouse Anti-Human CD11c (MCA2087PE) and FITC conjugated Mouse Anti-Human HLA DP/DQ/DR (MCA477F). All experiments performed on human peripheral blood mononuclear cells in the presence of Human SeroBlock (BUF070A).
Mouse Markers
The H-2 complex genes encode for proteins of the mouse MHC. There are three main classes of H-2 genes, class I (H2-K, H2-D, Q, H2-T18), class II (H2-I) and class III (H2-S). The H-2 genes are highly polymorphic and involved in many processes in addition to antigen presentation such as complement components and immune response. Table 2 shows a number of the different markers available to detect the different molecules of H-2.
Table 2. Mouse H-2 markers and antibodies.
H-2 |
Antibody |
Clone |
|---|---|---|
|
MHC class I |
|
|
|
MHC class I |
2G5 |
|
|
MHC class I H-2b/d/p/q/w16 |
ER-HR52 |
|
|
MHC class I H-2Dd H-2Kv H-2k/q/s |
ER-MP42 |
|
|
MHC class I H-2Kb |
AF6-88.5 |
|
|
MHC class II |
|
|
|
MHC class II H-2I-Ak/d/b/q/r |
ER-TR3 |
|
|
MHC class II H-2I-Ak/s/r |
ER-TR2 |
|
|
MHC class II I-A/I-E |
M5/114.15.2 |
Rat Markers
In rats the MHC is classified as RT1-A class I and RT1-B class II. Additional gene regions have been identified to be associated with RT1-B; RT1-H and RT1- D. Table 3 shows a number of the different markers available to detect the different molecules of RT1-A class I and RT1-B class II MHC.
Table 3. Rat MHC markers and antibodies.
RTL |
Antibody |
Clone |
|---|---|---|
|
MHC class I |
|
|
|
RT1A |
OX-18 |
|
|
RT1Ac |
OX-27 |
|
|
MHC class II |
|
|
|
RT1B |
OX-6, F17-23-2 |
|
|
RT1Bu/I |
OX-3 |
Featured Key Marker RT1B (MHC Class II)
Mouse Anti-Rat MHC Class II RT1B Antibody, clone OX-6 recognizes a monomorphic determinant of the rat RT1B MHC class II antigen present on B lymphocytes, dendritic cells, some macrophages and certain epithelial cells. Rat markers RT1B and CD45RC have been used in flow cytometry (Figure 3), showing the MHC Class II positive B cell populations.
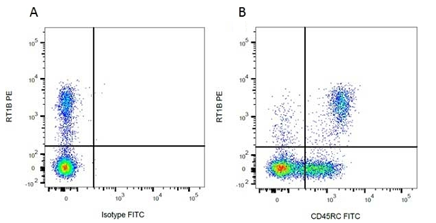
Fig.3. Flow cytometry analysis of rat RT1 marker RT1B. A, RPE conjugated Mouse Anti-Rat RT1B (MCA46PE) and FITC conjugated Mouse IgG1 Isotype Control (MCA1209F). B, RPE conjugated Mouse Anti-Rat RT1B (MCA46PE) and FITC conjugated Mouse Anti-Rat CD45RC (MCA53FT). All experiments performed on red cell lysed rat blood gated on mononuclear cells.
Veterinary Markers
Markers to MHC Class I and II are also available against many veterinary species, such as cattle, sheep, chicken and horse. Table 4 shows a number of the different markers available to detect the MHC different molecules of different veterinary species.
Table 4. Veterinary MHC markers and antibodies.
|
Target Species |
Antibody |
Clone |
|---|---|---|---|
|
MHC class I |
|
|
|
|
|
Bovine |
IL-A88 |
|
|
|
Chicken |
F21-2 |
|
|
|
Horse |
CVS22 |
|
|
|
Rabbit |
73-2 |
|
|
|
Sheep |
VPM19 |
|
|
MHC class II |
|
|
|
|
MHC class II |
Cat |
PF8J-9B, PF6J-6D |
|
|
DQ |
Bovine |
CC158 |
|
|
|
Rabbit |
2C4 |
|
|
|
Sheep |
49.1, 28.1 |
|
|
DR |
Bovine |
CC108 |
|
|
|
Sheep |
37.68 |
|
|
Monomorphic |
Bovine |
IL-A21 |
|
|
|
Cat |
Vpg3 |
|
|
|
Chicken |
21-1A6 |
|
|
|
Dog |
YKIX334.2, CA2.1C12 |
|
|
|
Guinea Pig |
CI.13.I |
|
|
|
Horse |
CVS20 |






