Characterization of Critical Reagents for Ligand Binding Assays
-
Bioanalytical Antibodies
-
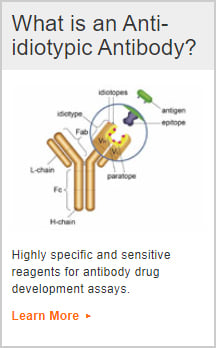
Anti-idiotypic Antibodies
- Why Choose Bio-Rad Anti-Idiotypic Antibodies
- Antibodies for Bioanalysis and Drug Monitoring
- Our Anti-Biotherapeutic Antibodies
- Biosimilar Antibodies for Research Use
- Anti-IgG Fc Antibodies
- Recombinant Fully-Human Immunoglobulin Isotype Controls
- Anti-Biotherapeutic Antibody Quality Control and Characterization
- Characterization of Critical Reagents for Ligand Binding Assays
-
Anti-idiotypic Antibodies
s
s
s
Overview
Ligand binding assays are utilized throughout the large molecule drug development process. However their robustness, accuracy and reproducibility depend on the quality of critical reagents. The unique characteristics of these reagents are crucial to assay performance, and require careful characterization. To ensure their consistent characterization in bioanalytical laboratories, a team of pharmaceutical scientists have outlined recommendations and best practices for this process, particularly during the early stages of drug development.
Drug development relies on ligand binding assays (LBAs), which are bioanalytical procedures widely used to measure the immunogenicity of biotherapeutic molecules, and determine drug concentrations for pharmacokinetic analyses. The accuracy and performance of LBAs, however, depends on the quality of key reagents, called critical reagents. The Global Bioanalysis Consortium (GBC) and the European Medicines Agency classify critical reagents as analyte specific or binding reagents, specifically antibodies; peptides; engineered proteins; antibody, protein and peptide conjugates; reagent drugs; aptamers and anti-drug antibody (ADA) reagents including positive and negative controls (King et al. 2014). In 2014, the GBC expanded this definition to include biological matrices, in some instances (King et al. 2014). Utilizing poorly characterized critical reagents in LBAs could lead to inaccurate conclusions and delays in the drug development process. It is therefore necessary to properly characterize them early in the drug development program. However, their characterization and management vary between bioanalytical laboratories.
Categories of critical reagents used in ligand binding assays
- Antibodies
- Peptides
- Engineered proteins
- Chemically synthesized molecules
- Antibody, protein and peptide conjugates
- Complex biologics
- Solid supports and matrices
Best practices and recommendations for critical reagent characterization
The initiative to generate a consensus within the pharmaceutical industry regarding the expectations and operations of a bioanalytical laboratory originated from a 2009 American Association of Pharmaceutical Scientists workshop on the Twenty-First Century Bioanalytical Laboratory. Following up on this initiative, O’Hara et al. (2012) developed and published a set of recommendations and best practices for the life cycle management, characterization and supply of critical reagents used in LBAs.
This article summarizes the guidelines for characterizing critical reagents, particularly during the early stages of drug development. In this context, characterization refers to the process of identifying the physicochemical attributes of these reagents (O’Hara et al. 2012). Characterizing critical reagents is essential for ensuring optimal assay performance over time as these attributes can serve as a guide for: 1) identification of the root causes of assay performance problems, 2) determination of when a reagent is deteriorating and 3) the generation of new reagents and screening of different lots.
To enhance consistency in the industry and guide decision making, O’Hara et al. (2012) propose the following recommendations and best practices for characterizing critical reagents in LBAs.
Recommended critical reagent characteristics to measure
Sensitivity
- Concentration (antibody titer)
- Binding activity
- Potency
- Binding kinetics and affinity determination
- Neutralization activity
- Aggregation level
Specificity
- Cross-reactivity
Reproducibility
- Molecular weight
- Purity
- Isotype (for monoclonal antibodies)
- Formulation buffer selection
- Isoelectric point
- Conjugate incorporation ratio
- Endotoxin level
- Protein A level
- Host cell protein level
- Stability
- Bovine IgG level
- Functional assays
-
It is important to generate a basic characterization profile for each critical reagent that includes assessment of concentration (antibody titer), binding activity, aggregation, purity level and molecular weight.
-
Include purification assays for protein reagents during early stages of drug development to reduce the impact of impurities, which could affect stability during long term storage.
-
Verify purified reagents using methods such as SDS-PAGE and SEC to confirm purity and monodispersity prior to use in the desired LBA.
-
As drug development progresses, express monoclonal antibody reagents in characterized mammalian cell lines to ensure their genetic stability and consistency across reagent lots.
- Where appropriate, consider assessing optional characterization parameters such as determining protein A levels in a protein purified using a protein A column, bovine IgG levels from tissue culture expansion, and residual host cell protein levels.
Antibody critical reagents developed by Bio-Rad
Bio-Rad develops highly specific, high affinity anti-biotherapeutic antibodies to support preclinical research, clinical trials and patient monitoring for innovator and biosimilar products. These anti-biotherapeutic antibodies for ADA and PK assay development are generated using the HuCAL® recombinant monoclonal antibody library and an improved, proprietary method of antibody phage display, resulting in fully human antibodies in Fab and full length immunoglobulin (Ig) formats. Integral to the process is in vitro guided selection, which enables the generation of highly specific inhibitory and non-inhibitory anti-idiotypic antibodies and specialized drug-target complex binders. The antibody sequence is always known and stored for long term security, and the methods used are well established, proven and highly reproducible. Fab antibodies are expressed in E. coli and full length Ig formats are expressed in a characterized human cell line using ultra-low bovine IgG FCS in the production method. Antibodies are then purified using affinity chromatography. The quality control and characterization process is thorough, consistent and incorporates several of the relevant best practices outlined by O’Hara et al. (2012). Every new antibody and subsequent lots are subject to a strict quality control procedure to ensure a high quality product every time, helping the customer with critical reagent life cycle management, characterization and supply, and the maintenance of optimal assay performance over time.
Summary
LBAs are instrumental in the drug development process. They utilize a set of reagents of which some are deemed critical to the performance of the assay. These critical reagents directly impact the quality and reproducibility of LBAs and consequently can delay the drug development process if they are of poor quality. Because they are so important to the success of LBAs, careful characterization of critical reagents should be performed, whether purchased or produced in-house. Best practices and recommendations for characterizing critical reagents are not fully standardized within the pharmaceutical industry. A team of scientists has addressed this concern by providing the best practices for critical reagents characterization. These guidelines focus on generating a characterization profile for each critical reagent that will ultimately be useful for ensuring the long-term robustness of LBAs.
Bio-Rad incorporates best practices for quality control and analytical antibody batch characterization as part of the manufacturing and product development of the anti-biotherapeutic antibody portfolio. These antibodies are being adopted as critical reagents in assays carried out as part of bioanalytical testing services provided by global contract research organizations, and in pre-clinical and clinical studies in pharmaceutical companies.
Related Topics
References
- King LE et al. (2014). Ligand binding assay critical reagents and their stability: Recommendations and best practices from the global bioanalysis consortium harmonization team. AAPS J 16, 504-515.
- O’Hara DM et al. (2012). Ligand binding assays in the 21st century laboratory: Recommendations for characterization and supply of critical reagents. AAPS J 14, 316-328.