Inflammation
Quick Pick Phenotype Marker Finder

Click through and see at a glance the key markers for immune cell phenotyping and for identifying specific stages of apoptosis.
s
Interactive Tool

Human and mouse immune cell marker databases. All the facts at your fingertips from cell lineage to marker proteins, discover for yourself.
s
Inflammation Overview
Inflammation is an evolutionarily conserved physical process, affecting any part of the body in which the immune system senses an infection or injury. The five classic signs of inflammation are redness, heat, swelling, pain, and loss of function, although not every inflammatory response shows all five signs (Hawiger and Zienkiewicz 2019). Figure 1 depicts the initiation of an inflammatory response. Inflammation serves as a short-term response to assist the immune system with eliminating a pathogen or healing a wound, but both chronic and allergic inflammation are common and can be harmful.
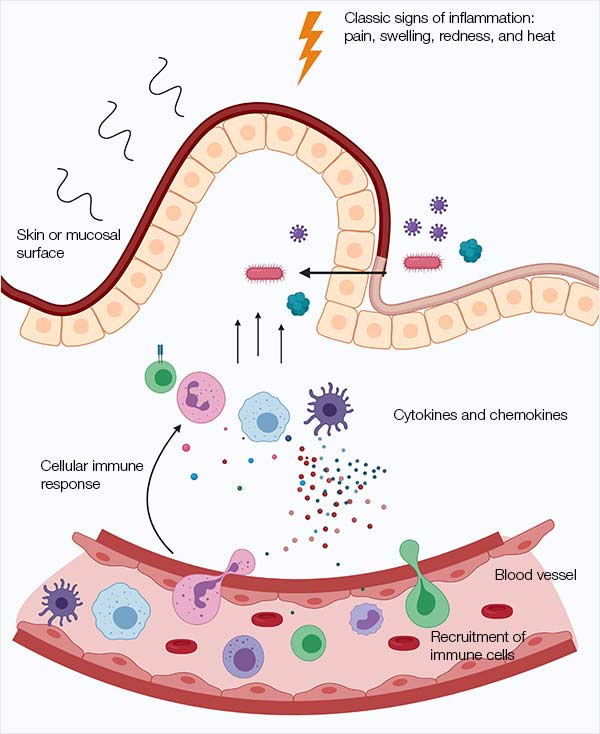
Fig. 1. Initiation of inflammation.
Our cells can produce an inflammatory response tailored to a specific type of threat ─ a viral infection for example can lead to inflammation which upregulates specific antiviral agents or wound healing responses are produced following a skin abrasion (Koh and DiPietro 2011). This is controlled by cell signaling focused around the inflammatory master regulator NF-κB, whereby a range of stimuli can result in a multitude of specific outcomes. Broadly, the five types of inflammation are categorized as microbial, autoimmune, allergic, metabolic, and physical (Hawiger and Zienkiewicz 2019). However, they are not all helpful. For research into microbial inflammation, review Bio-Rad’s range of infectious disease antibodies and antigens.
If the immune system is acting correctly, an inflammatory response will only be initiated because of an actual threat. However, the immune system can trigger inflammation in reaction to an unharmful substance, such as grass, pollen or peanuts, underlying allergic inflammation, or against self, termed autoimmune inflammation. In other situations, inflammation can continue for a long time and cause damage. Chronic inflammation causes the symptoms of rheumatoid arthritis, inflammatory bowel disease (IBD), and asthma (Kuenzig et al. 2018). Some cancers can create microenvironments that sustain inflammation, creating beneficial conditions for the tumor to survive and grow (Multhoff et al. 2011).
At the heart of the inflammatory response lies a complex web of molecular interactions. Immune cells operate at the command of precise cytokine levels, orchestrated by vast signaling networks. However, the larger and more complex a network, the more likely it is to go wrong.
The Process of Inflammation
Pattern recognition receptors (PRRs) provide a mechanism by which a cell can sense danger, recognizing pathogen-associated molecular pattern molecules (PAMPs) and damage‐associated molecular pattern molecules (DAMPs). PAMPs are present in pathogenic microorganisms, while DAMPs are compounds produced by the body’s own cells in response to trauma, ischaemia, and tissue damage (Tang et al. 2012). Membrane-bound PRRs include Toll-like receptors (TLRs), a group of proteins that dimerize to recognize distinct molecular patterns (Sabroe et al. 2008). Learn more about PPRs; their classification and the antibodies available to detect them, or find out about their role in instigating cell death during infection.
When an innate immune cell first senses a PAMP or a DAMP, it triggers a series of chemical changes. The cell may start producing cytokines – small secreted messenger proteins – which communicate the danger to immune cells, so that an infection can be eradicated, or a wound can be healed. Cytokines can act on the cells that secrete them (autocrine action), on nearby cells (paracrine action), or on distant cells (endocrine action). Immune cells including macrophages, B cells, T cells, neutrophils, and mast cells both produce and respond to cytokines. They are recruited to the site of infection or injury and can help to maintain an inflammatory response or restore homeostasis (Zhang and An 2007).
NF-κB is a coordinator of inflammation
One of the most important proteins in the inflammatory response is the transcription factor nuclear factor-kappaB (NF-κB). Rather than being a single protein, NF-κB represents a family of five structurally-related proteins which regulate hundreds of genes involved with inflammation and immunity. In basal conditions, NF-κB is bound in the cytoplasm by inhibitor kappaB alpha (IκBα). When the cell senses a threat or an inflammatory cytokine, such as tumor necrosis factor alpha (TNF-α), IκBα is degraded by the proteasome, releasing NF-κB from inhibition. NF-κB then translocates to the nucleus where it activates transcription of genes that initiate and maintain an inflammatory response (Mussbacher et al. 2019). NF-κB acts as a biological oscillator; able to promote transcription of its own inhibitor (IκBα), it has been observed to translocate in and out of the nucleus with a steady period, in sync with IκBα levels rising and falling through synthesis and degradation. NF-κB appears to be able to regulate gene expression depending on the pattern of stimulation by TNF-α, giving it a role as a central coordinator of the inflammatory response (Ashall et al. 2009).
NF-κB is important for inducing inflammatory genes in innate immune cells, including macrophages, dendritic cells, and neutrophils. Inflammation requires components of the adaptive immune system too, for example, T helper (Th)1 and Th17 cells mediate inflammatory responses. Accordingly, NF-κB also plays a role in the adaptive immune system, regulating the activation, differentiation, and function of inflammatory T cells (Liu et al. 2017).
Bio-Rad offers a range of antibodies to various subunits of NF-κB, for use in applications such as ELISA, imaging, and western blotting. Review the NF-κB antibodies available, which include PrecisionAb Western Blotting Antibodies that have been rigorously tested on multiple cell and tissue lysates.
Cellular response to infection during inflammation
Cytokines are released during an inflammatory response and attract immune cells to the site of damage. Table 1 lists important cytokines involved during inflammation. Leukocytes, in particular neutrophils, are one of the first cell types recruited by inflammation. They can target microorganisms directly but also play a wider role in inflammatory coordination, helping T cells to attract other immune cells. Monocytes are recruited after neutrophils, and can differentiate into macrophages and dendritic cells as needed.
Table 1. Cytokines involved during inflammation (Kany et al. 2019).
Click on the links to find out the antibodies available to detect these cytokines.
Cytokine |
Overview |
|---|---|
|
Multifunctional pro-inflammatory cytokine, produced by a variety of cells including activated macrophages, monocytes, and keratinocytes. |
|
|
Multifunctional pro-inflammatory cytokine, produced by activated T cells, macrophages, endothelial cells, fibroblasts, and osteoblasts. IL-6 signals through a membrane bound receptor complex consisting of the ligand-binding IL-6Rα chain (CD126) and the signal-transducing component gp130 (CD130), but can also stimulate cells expressing gp130 through a soluble IL-6R. Diverse biological properties include the stimulation of B cell differentiation and subsequent immunoglobulin production and promotion of hybridoma growth. |
|
|
Pro-inflammatory CXC chemokine designated CXCL8, produced by endothelial cells and monocytes. IL-8 signals through the CXCR1 and CXCR2 receptors, and acts as a major chemoattractant for neutrophils, T cells, and basophils. |
|
|
Immunosuppressive cytokine originally known as cytokine synthesis inhibiting factor (CSIF). IL-10 is expressed by activated T cells, mast cells, activated B cell, and macrophages. IL-10 inhibits the secretion of a variety of pro-inflammatory cytokines including TNF-α, IL-1, IL-2, IL-3, and IL-8 and is chemotactic for CD8+ T cells. |
|
|
IL-33 is abundantly expressed in endothelial venules found in tonsils, peyers patches, and mesenteric lymph nodes. IL-33 signals through interaction with the ST2 and toll-interleukin 1 (IL-1) receptors, and is known to act as an immunoregulator of T helper 2 (Th2) cell cytokine synthesis. |
|
|
Multifunctional pro-inflammatory cytokine produced by macrophages, T and B lymphocytes, activated monocytes, and fibroblasts. TNF-α exists in both a transmembrane and mature soluble form and has many biological properties, including activation of transcription factor NF-kappaB, the induction of apoptosis in various tumor cell lines, and the stimulation of cell differentiation and proliferation under certain conditions. TNF-α also plays a role in inflammation, septic shock, autoimmune disease, and rheumatoid arthritis. |
Inflammatory cytokines (IL = interleukin, TNF-α = tumor necrosis factor alpha).
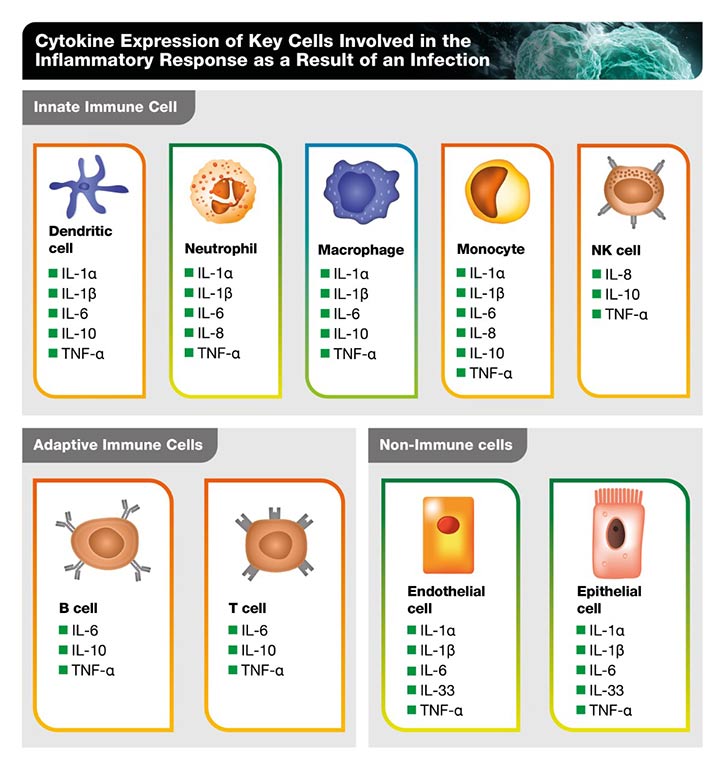
Macrophages are ubiquitous, critical regulators of the inflammatory response. They are a type of phagocyte, capable of engulfing and destroying pathogens and apoptotic cells, thereby maintaining healthy tissue. The role of macrophages is context-dependent, for example, they can maintain an inflammatory response while also help suppress inflammation, clear debris, and support any damaged tissues once the pathogen is cleared (Murray and Wynn 2011). Table 2 shows the cytokines expressed by the main cells involved in the inflammatory response.
For more information about cytokine involvement in the immune response, resulting in a cytokine storm, read our blog “Navigating the Deadly Cytokine Storm”.
Table 2. Cytokine expression of key cells involved in the inflammatory response as a result of an infection (Kany et al. 2019).
Click on the links for further information about these innate and adaptive immune cells and to discover the antibodies available to these cytokines.
Markers of Inflammation
Markers of inflammation are used to detect acute inflammation that might indicate a specific disease and also to assess treatment response. Raised levels of inflammatory markers can indicate the probability of infections, autoimmune conditions, and cancers. Where levels are normal, certain conditions can be ruled out. Although they are valuable for indicating diseases, inflammatory markers are not specific enough to allow diagnosis of serious underlying disease (Watson and Hamilton 2012).
The most common inflammatory markers are C-reactive protein, erythrocyte sedimentation rate, and procalcitonin. Although other markers of inflammation are useful in certain circumstances (Table 3).
Table 3. Inflammatory markers (Pearson 2020, Targońska-Stępniak and Majdan 2014, Monastero and Pentyala 2017, Mackiewicz and Mackiewicz 1995, labtestsonline.co.uk, Adamczyk-Sowa et al. 2016, D’Angelo 2013, Wang et al. 2001).
Click on the links to find out the antibodies available to detect these markers of inflammation.
Marker |
Application |
|---|---|
|
C-reactive protein (CRP) |
Commonly used early indicator of acute systemic inflammation, which can also reflect the severity of inflammatory insult. |
|
Erythrocyte sedimentation rate (ESR) |
Indirect measurement of plasma protein concentrations affected by numerous disease states. Levels of ESR are dependent on multiple proteins with a range of half-lives, so rates rise and fall slower than CRP. |
|
Procalcitonin (PCT) |
Marker of bacterial infection, severe viral infection, pancreatitis, tissue trauma, and certain autoimmune disorders. Useful in the diagnosis of sepsis. |
|
Acute phase protein released in response to inflammation or infection. Concentration increases dramatically during acute infection and injury. |
|
|
Small proteins including interleukins, chemokines, interferons, and tumor necrosis factors with varying roles in inflammation and immunity. |
|
|
Alpha-1-acid glycoprotein |
Serum acute phase glycoprotein. Relative levels of the protein’s four glycoforms can indicate a range of different conditions. |
|
Plasma viscosity |
Measurement of thickness of the liquid part of blood, affected by presence of proteins produced in response to infection or inflammation. |
|
One of the main proteins in metabolism and distribution of copper in blood serum, and appears to act as an antioxidant. Positive-phase protein, meaning that its level changes in acute and chronic inflammation. |
|
|
Hepcidin |
Regulator of iron metabolism produced by the liver. Iron deficiency can be indicated by reduced hepcidin levels. Levels of hepcidin are often abnormally high during inflammation, such as during sepsis or in patients with IBD. |
|
Acute phase protein induced by inflammation, which can bind hemoglobin and act as an antioxidant. |
Dysregulated Inflammation Underlies Many Chronic Diseases
Inflammation should terminate once the stimulus has been removed, long-term inflammation can be harmful. This is of concern if the stimulus is constant, or if the inflammatory response can’t otherwise be stopped. Low-grade chronic inflammation can be caused by a range of factors including diet, disturbed sleep, physical inactivity, and exposure to pollution. These factors can be site-specific; tobacco smoking and asbestos exposure inflame the lungs and excessive alcohol consumption can cause chronic inflammation in the liver. Long-term infections can also cause chronic inflammation. Infection with the bacteria Helicobacter pylori leads to chronic inflammation in the stomach (Lamb and Chen 2013). The hepatitis C virus causes liver inflammation and increases the risk of hepatocellular carcinoma (HCC), a type of liver cancer (Yi and Yuan 2017).
During systemic inflammation, the immune components activated are often distinct from those observed during an acute immune response, meaning that the biomarkers of acute inflammation (IL-6, TNF-α, IL-1β, CRP) are not a robust indicator of systemic chronic inflammation. Long-term, low-grade inflammatory responses can lead to a breakdown of immune tolerance, as well as major alterations in both normal cell physiology and tissue and organ function (Furman et al. 2019). Chronic inflammation plays a role in many cardiovascular, pulmonary and autoimmune diseases, cancer, diabetes, arthritis, Alzheimer’s disease, and many more conditions.
Dysregulated NF-κB signaling is a hallmark of many chronic inflammatory diseases including rheumatoid arthritis (RA), inflammatory bowel disease (IBD), multiple sclerosis, atherosclerosis, type 1 diabetes, and asthma. Given that NF-κB is a central regulator of inflammation with roles in innate immune cells, T cells, and the inflammasome, there are an astounding number of ways in which this protein can be dysregulated and perturb the inflammatory response. Accordingly, NF-κB plays different roles in different disease states. Targeting the NF-κB pathway represents a mechanism to modulate chronic inflammation in many disease states, and significant research is ongoing (Liu et al. 2017). However, immunosuppressive drugs for the control of chronic inflammatory disease can have long-term adverse events that must be carefully managed (Hsu and Katelaris 2009).
Chronic inflammation, induction of proinflammatory mediators, and accumulation of immune suppressor cells can result in immunosuppression. Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of immature myeloid cells. Their populations are massively expanded in autoimmune diseases including IBD, and they disrupt normal homeostasis by interrupting immune cell maturation (Wang and DuBois 2015). Immunosuppression also plays a role in cancer, partly via MDSCs. Since the immune system plays an important role in removing precancerous cells, an immunosuppressive microenvironment can favor cancer development (Wang and DuBois 2015).
Inflammation and cancer
Up to 93% of human cancers are nonhereditary, and are instead caused by interaction with environmental factors in some way (Parsa 2012). Furthermore, up to 25% of human cancers are thought to be related to chronic inflammation or to viral and bacterial infections (Multhoff et al. 2011). Chronic inflammation fosters a niche that permits upregulated cell proliferation and an increased frequency of DNA mutations, creating a perfect environment for cancer cells to survive and proliferate. As a result, cancer can result from sites of chronic inflammation after many years of low-level damage (Singh et al. 2019).
Within a chronic inflammatory microenvironment, macrophages produce high levels of reactive oxygen species (ROS) and nitrogen species to fight infection. While these molecules are important for fighting acute infection, they become harmful in a chronic inflammatory environment when tissue damage and proliferation constantly occur. Constant production of inflammatory cytokines can result in DNA damage by inhibiting DNA repair (Singh et al. 2019). Essentially, cells and tissues focus on trying to clear a real or inflammatory threat using inflammation, and in doing so can pay less attention to maintaining genetic integrity.
In addition to NF-κB, other transcription factors involved with cancer and inflammation include STAT-3 and HIF-1. Cytokines particularly important in this link include IL-1, IL-6, and TNF, modulating a pro-inflammatory environment. The link between inflammation and cancer is not straightforward. While inflammatory environments can promote carcinogenesis, malignant transformation, tumor growth, and metastasis, inflammation can also help the immune system to limit tumor growth. Such a complex system allows different outcomes under different conditions (Multhoff et al. 2011).
Targeting inflammation as disease treatment
Given the contribution of lifestyle factors to chronic inflammation, it is unsurprising that clinical trials have shown that modifying these factors can have beneficial inflammatory outcomes (Todoric et al. 2016). Furthermore, many pharmaceutical interventions aim at modulating the inflammatory response for treatment of chronic conditions. An everyday example is the use of topical corticosteroids, hydrocortisone, and prednisone for example, for treating eczema flare-ups (informedhealth.org). Inhibitors of TNF-α are commonly used to treat rheumatoid arthritis (Farrugia and Baron 2016). Both pro- and anti-inflammatory treatments can be used as cancer therapies depending on the context; sometimes a treatment focuses on reducing the chronic inflammation that facilitates tumor growth, and at other times it aims at stimulating the immune system to destroy cancerous cells (Ritter and Greten 2019). The number of ways in which the inflammatory response can be regulated is truly remarkable, but the unmet need to better address countless inflammatory conditions shows the complexity of this system.
Antibodies to Study Inflammation
Bio-Rad offers an extensive selection of immunology antibodies that cover both innate and adaptive immunity. Performance guarantee in key immunological applications including flow cytometry, ELISA, immunohistochemistry, immunofluorescence, and western blotting.
To support you, Bio-Rad has developed a comprehensive application resources library that provides essential information needed to design your experiments, select the best marker, and identify the right antibody with ease.
References
- Adamczyk-Sowa et al. (2016). Changes in serum ceruloplasmin levels based on immunomodulatory treatments and melatonin supplementation in multiple sclerosis patients. Med Sci Monit 22, 2484–2491.
- Ashall et al. (2009). Pulsatile stimulation determines timing and specificity of NF-kappaB-dependent transcription. Science 324, 242–6.
- D’Angelo (2013). Role of hepcidin in the pathophysiology and diagnosis of anemia. Blood Res 48, 10-15.
- Farrugia and Baron (2016). The role of TNF-α in rheumatoid arthritis: a focus on regulatory T cells. J Clin Transl Res 2, 84–90.
- Furman et al. (2019). Chronic inflammation in the etiology of disease across the life span. Nature Medicine 25, 1822–1832.
- Hawiger and Zienkiewicz (2019). Decoding inflammation, its causes, genomic responses, and emerging countermeasures. Scand J Immunol 90, e12812.
- Hsu and Katelaris (2009). Long-term management of patients taking immunosuppressive drugs. Aust Prescr 32, 68–71.
- InformedHealth.org. Eczema: Steroids and other topical medications. 2017 Feb 23. Available from: https://www.ncbi.nlm.nih.gov/books/NBK424899/
- Kany et al. (2019). Cytokines in Inflammatory Disease. In J Mol Sci 20, 6008.
- Kuenzig et al. (2018). Co-occurrence of asthma and the inflammatory bowel diseases: a systematic review and meta-analysis. Clin Transl Gastroenterol 9, 188.
- Lamb and Chen (2013). Role of the helicobacter pylori-induced inflammatory response in the development of gastric cancer. J Cell Biochem 114, 491–497.
- Liu et al. (2017). NF-κB signaling in inflammation. Signal transduction and targeted therapy 2, 17023.
- Koh and DiPietro (2011). Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med 13, e23.
- Mackiewicz and Mackiewicz (1995). Glycoforms of serum Alpha 1-acid glycoprotein as markers of inflammation and cancer. Glycoconj 12, 241–7.
- Monastero and Pentyala (2017). Cytokines as biomarkers and their respective clinical cutoff levels. Int J Inflam 4309485.
- Multhoff et al. (2011). Chronic inflammation in cancer development. Front Immunol 2, 98.
- Mussbacher et al. (2019). Cell type-specific roles of NF-κB linking inflammation and thrombosis. Front Immunol 4, 85.
- Murray and Wynn (2011). Protective and pathogenic functions of macrophage subsets. Net Rev Immunol 11, 723–737.
- Newton and Dixit (2012). Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol 4, a006049.
- Parsa (2012). Environmental factors inducing human cancers. Iran J Public Health 41, 1–9.
- Pearson, last modified June 2020. Inflammatory markers. Available from: https://arupconsult.com/content/inflammatory-markers
- Plasma viscosity, last modified 14 November 2018. Available from: https://labtestsonline.org.uk/tests/plasma-viscosity
- Ritter and Greten (2019). Modulating inflammation for cancer therapy. J Exp Med 216, 1234–1243.
- Sabroe et al. (2008). The role of TLR activation in inflammation. J Pathol 214, 126–35.
- Singh et al. (2019). Inflammation and cancer. Ann Afr Med 18, 121–126.
- Tang et al. (2012). PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev 249, 158–175.
- Targońska-Stępniak and Majdan (2014). Serum amyloid A as a marker of persistent inflammation and an indicator of cardiovascular and renal involvement in patients with rheumatoid arthritis. Mediators of Inflammation 793628.
- Todoric et al. (2017). Targeting inflammation in cancer prevention and therapy. Cancer Pres Rev 9, 895–905.
- Wang and DuBois (2015). Immunosuppression associated with chronic inflammation in the tumor microenvironment. Carcinogenesis 36, 1085–1093.
- Wang et al. (2001). Haptoglobin, an inflammation-inducible plasma protein. Redox Rep 6, 379–85.
- Watson and Hamilton (2012). Raised inflammatory markers. BMJ 344:e454.
- Yi and Yuan (2017). Hepatitis C virus-associated cancers. Adv Exp Med Biol 1018, 129–146.
- Zhang and An (2007). Cytokines, inflammation and pain. Int Anesthesiol Clin 45, 27–37.



