Our Antibody Validation Principles
Importance of Antibody Validation
Poor reproducibility of results is a widespread issue in scientific research (Goodman 2018). A survey carried out by Nature in 2016 highlighted that greater than 70% of researchers have struggled to reproduce experiments carried out by another scientist, and many researchers have encountered issues reproducing their own data. Irreproducible data holds back scientific progress, as well as hampering individual research efforts (Baker 2016). It is imperative that observations can be repeated to establish biological truths, like interacting proteins and identification of cell types.
Irreproducible data can often be attributed to poorly validated antibodies, not working as intended. Issues like cross-reactivity, batch-to-batch variability, and antibody use in inappropriate applications can lead to differing results (Baker 2015). Furthermore, poor antibody choices can lead to paper retractions and further research being built on false premises.
Antibody Validation Principles and Methods
“Validation is the experimental proof and documentation that a specific antibody is suitable for an intended application or purpose”. (Weller 2018)
Well-characterized antibodies are essential to address the “reproducibility crisis” as they have consistent performance and generate robust data. With this in mind, the research community is taking steps to raise the profile of antibody validation, including the formation of the International Working Group for Antibody Validation (IWGAV) and the publication of recommendations for antibody validation standards, known as the five pillars (Uhlen et al 2016). Bio-Rad is committed to evolving its validation standards and we constantly review our in-house testing in line with the five pillar recommendations.
We have introduced the following additional validation methods:
- Knockout (KO) validation
- Knockdown (siRNA) validation
- Immunoprecipitation followed by mass spectrometry (IP-MS)
Bio-Rad’s antibodies have been tested to work in the applications stated on the product datasheets, helping you select appropriate antibodies. Selecting well characterized antibodies, with resources to support their use, can reduce the necessary time to confirm their suitability for your assay. We provide detailed protocols and troubleshooting guides to enable optimal performance of our products, and should you encounter any performance issues, our technical support team will help you investigate. We conduct rigorous testing of all new products to ensure they meet our performance standards. If a product should fall short of our high standards, we will withdraw it from sale.
As Antibody Manufacturers, Product Quality Is Essential
![]()
Bio-Rad conducts rigorous in-house testing to guarantee that our antibodies meet our internal benchmarks and perform in their designated applications as expected. Our stringent quality control process is recognized by ISO9001:2015 certification at our manufacturing sites in Kidlington, Oxfordshire, UK, and Neuried, Germany.
We clearly display recommended applications for every antibody on our website and product datasheet.
In addition, the Bio-Rad Quality and Performance Guarantee ensures that if a catalog antibody batch doesn’t meet the specifications as described on the datasheet, and the issue cannot be resolved by our dedicated technical support team, we will replace the antibody free of charge or offer a credit.
Transparency Is Key
![]() Sharing your knowledge can help other scientists find the best antibodies for their research. Your feedback is therefore vital to help other researchers understand how an antibody has performed in different applications and species. Including extensive details of antibodies used in your publications such as vendor, clone ID, and experimental conditions will help other scientists verify your findings. These details also help validate that antibody for the published application.
Sharing your knowledge can help other scientists find the best antibodies for their research. Your feedback is therefore vital to help other researchers understand how an antibody has performed in different applications and species. Including extensive details of antibodies used in your publications such as vendor, clone ID, and experimental conditions will help other scientists verify your findings. These details also help validate that antibody for the published application.
Bio-Rad’s PrecisionAb Antibody Range
 In line with recommendations by the International Working Group for Antibody Validation (Uhlen et al. 2016), we are implementing methods to validate our antibodies. Only antibodies meeting all of our stringent criteria are released as PrecisionAb Antibodies.
In line with recommendations by the International Working Group for Antibody Validation (Uhlen et al. 2016), we are implementing methods to validate our antibodies. Only antibodies meeting all of our stringent criteria are released as PrecisionAb Antibodies.
![]() Watch the video to find out more.
Watch the video to find out more.
Learn about the importance of antibody validation to ensure specificity. Discover the comprehensive validation methods implemented by Bio-Rad, including CRISPR-Cas9 gene editing, siRNA, and immunoprecipitation followed by mass spectrometry.
Antibodies Verified for Flow Cytometry
In-house manufactured antibodies that have “flow cytometry” indicated on the datasheet have been assessed to ensure application suitability. This involves testing the antibody for performance in flow cytometry using a relevant cell line or primary cells known to express the marker under appropriate conditions.
We use appropriate controls including unstained cells, isotype staining, secondary only controls, dual stains, and positive and negative controls (Figure 1 and 2). We also test different dilutions of an antibody to determine the correct antibody concentration. Antibodies are tested for cross-reactivity where relevant. It is also critical that the flow cytometer functions appropriately for reliable antibody validation in flow. We make sure that routine maintenance of our ZE5 Cell Analyzer is performed.
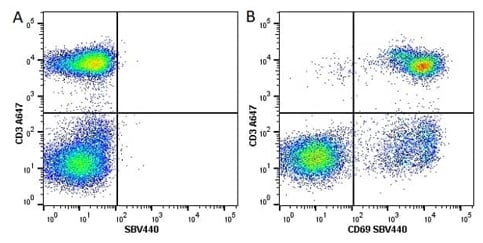
Fig. 1. Flow cytometry analysis of CD3 expression. A, Alexa Fluor 647 Conjugated Mouse Anti-Human CD3 (MCA463A647). B, Alexa Fluor 647 conjugated Mouse Anti-Human CD3 (MCA463A647) and StarBright Violet 440 Dye Conjugated Mouse Anti-Human CD69 (MCA2806SBV440). All experiments performed on human blood stimulated with Cell Stimulation Reagent (without Brefeldin A) (BUF076A) for 5 hr, gated on live, single lymphocytes, in the presence of 10% human serum. Data were acquired on the ZE5 Cell Analyzer.
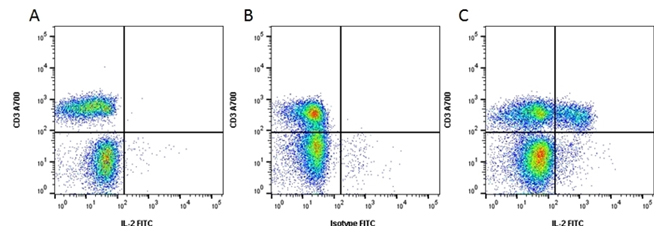
Fig. 2. Flow cytometry analysis of CD3 expression. A, unstimulated cells were stained with Alexa Fluor 700 Conjugated Mouse Anti-Human CD3 (MCA463A700) and FITC Conjugated Rat Anti-Human IL-2 (MCA1553F). B, cells stimulated with Cell Stimulation Reagent containing Brefeldin A (BUF077A) for 5 hr were stained with Alexa Fluor 700 Conjugated Mouse Anti-Human CD3 (MCA463A700) and FITC Conjugated Rat IgG2a (MCA6005F). C, cells stimulated with Cell Stimulation Reagent containing Brefeldin A (BUF077A) for 5 hr were stained with Alexa Fluor 700 Conjugated Mouse Anti-Human CD3 (MCA463A700) and FITC Conjugated Mouse Anti-Human IL-2 (MCA1553F). All experiments performed on red cell lysed human blood gated on lymphoid cells in the presence of 10% human serum. Data were acquired on the ZE5 Cell Analyzer.
Recombinant Antibodies
![]() Innovative HuCAL® technology enables in vitro generation of highly specific, fully human, recombinant monoclonal antibodies. The advantages of using recombinant antibodies include rapid delivery, lot-to-lot consistency, long-term secure supply, and importantly no immunization of animals. As a result, recombinant antibodies always offer the same optimal performance.
Innovative HuCAL® technology enables in vitro generation of highly specific, fully human, recombinant monoclonal antibodies. The advantages of using recombinant antibodies include rapid delivery, lot-to-lot consistency, long-term secure supply, and importantly no immunization of animals. As a result, recombinant antibodies always offer the same optimal performance.
References
- Baker M (2016). Many researchers fail to validate antibodies for their experiments. Nature. Accessed December 20, 2021.
- Baker M (2015). Reproducibility crisis: Blame it on the antibodies. Nature 521, 274–276.
- Goodman S L (2018). The antibody horror show: an introductory guide for the perplexed. New Biotechnology 45, 9–13.
- Uhlen M et al. (2016). A proposal for validation of antibodies. Nature Methods 13, 823–827.
- Weller MG (2018). Ten basic rules of antibody validation. Anal Chem Insights 13, 1177390118757462.