ELISA: Protocols
Overview
The enzyme-linked immunosorbent assay (ELISA) is a frequently used application which determines the concentration of an analyte in solution. The ELISA method is straightforward and rapid. With automated equipment the assay is easily scalable for set up in high-throughput and can handle a large number of samples in parallel, making it a popular choice for the evaluation of research and diagnostic targets.

General ELISA Protocols
Standard methods for different types of ELISA in 96-well microtiter plate format, including recommended buffers.
Protocols for use with Anti-Biotherapeutic Antibodies
Bio-Rad’s anti-biotherapeutic antibodies are ideal for the measurement of monoclonal antibody drugs and biosimilar products in PK and ADA assays.
We have developed a range of highly specific, recombinant anti-idiotypic antibodies with different binding modes and properties, enabling the development of bridging ELISA and antigen capture ELISA. Protocols are available for each type of PK assay and ADA assay for many of the anti-biotherapeutic products in our catalog.
PK and ADA Bridging ELISA Protocols
Bridging ELISA is a special case of a sandwich ELISA in which a dimeric or oligomeric antigen (most often an antibody in a sample) is detected by a capture and detection antibody. The antigen bridges the two specific antibodies. Bridging ELISAs are most frequently used for the detection of IgG, for example in pharmacokinetic (PK) or anti-drug antibody (ADA) assays.
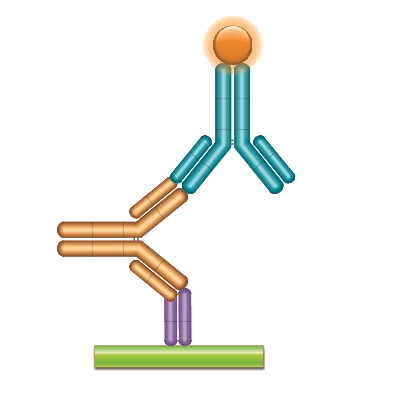
PK Bridging ELISA measuring free drug
Type 1 anti-idiotypic antibodies are inhibitory and are used to detect free drug
- Abatacept
- Adalimumab
- Atezolizumab
- Avelumab
- Avelumab (TZA041, TZA040P)
- Belatacept
- Bevacizumab
- Brentuximab vedotin
- Cemiplimab
- Cetuximab
- Daratumumab
- Denosumab
- Dupilumab
- Durvalumab
- Eculizumab
- Etanercept
- Evolocumab
- Golimumab
- Infliximab
- Ipilimumab
- Natalizumab
- Nivolumab
- Obinutuzumab
- Ocrelizumab
- Ocrelizumab (TZA044, TZA045)
- Omalizumab
- Palivizumab
- Panitumumab
- Pembrolizumab
- Pertuzumab
- Rituximab
- Secukinumab
- Tocilizumab
- Trastuzumab (HCA166, HCA168)
- Trastuzumab (HCA169, HCA176P)
- Trastuzumab (HCA169, HCA177P)
- Trastuzumab (HCA169, HCA270 IgG4)
- Ustekinumab (HCA208, HCA210)
- Ustekinumab (HCA209, HCA210)
- Vedolizumab
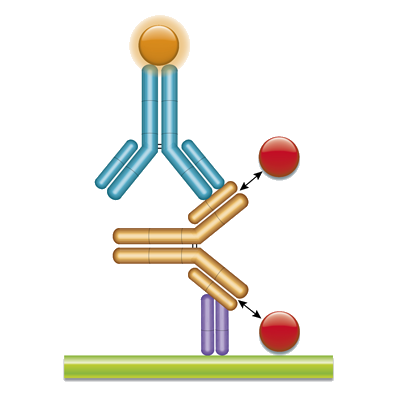
PK Bridging ELISA measuring total drug
Type 2 anti-idiotypic antibodies are non-inhibitory and enable the measurement of total drug – free, partially and fully bound
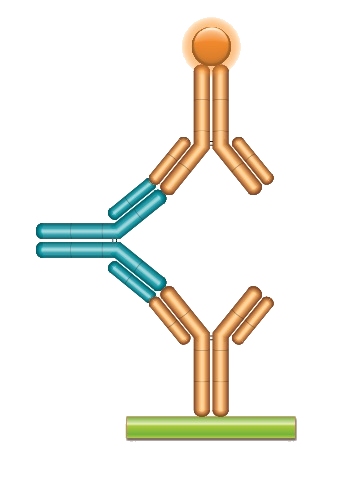
ADA Bridging ELISA
Type 1 anti-idiotypic antibodies are neutralizing and fully human; in immunoglobulin format they can be used as a control or calibrator as a surrogate for human ADAs
- Abatacept
- Adalimumab (IgG1)
- Adalimumab (IgE)
- Atezolizumab
- Avelumab
- Belatacept
- Bevacizumab
- Brentuximab vedotin
- Cemiplimab
- Certolizumab pegol
- Cetuximab
- Daratumumab
- Denosumab
- Dupilumab
- Durvalumab
- Eculizumab
- Etanercept
- Evolocumab
- Infliximab
- Ipilimumab
- Natalizumab
- Nivolumab
- Obinutuzumab
- Ocrelizumab
- Omalizumab
- Palivizumab
- Panitumumab
- Pembrolizumab
- Pertuzumab
- Ranibizumab
- Rituximab
- Secukinumab
- Tocilizumab
- Trastuzumab
- Ustekinumab
- Vedolizumab
Antigen Capture ELISA Protocols
A third type of antibody that only detects drug bound to its target antigen is available for some of our specificities.
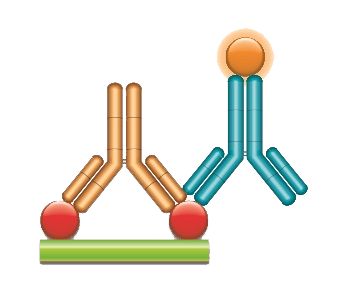
PK Antigen Capture ELISA measuring bound drug exclusively
A Type 3 antibody is used in an antigen capture ELISA to detect bound drug exclusively and avoids the bridging ELISA format
- Adalimumab
- Atezolizumab (TZA013)
- Atezolizumab (TZA013P)
- Avelumab
- Avelumab (TZA042P)
- Golimumab
- Durvalumab
- Ipilimumab (HCA331)
- Ipilimumab (TZA001P)
- Omalizumab
- Ranibizumab (HCA304, HCA306, HCA307)
- Ranibizumab (HCA316P)
- Secukinumab
- Trastuzumab




