Anti-Golimumab Antibodies
-
Bioanalytical Antibodies
-
Anti-idiotypic Antibodies
-
Our Anti-Biotherapeutic Antibodies
- Anti-Abatacept Antibodies
- Anti-Adalimumab Antibodies
- Anti-Alemtuzumab Antibodies
- Anti-Atezolizumab Antibodies
- Anti-Avelumab Antibodies
- Anti-Belatacept Antibodies
- Anti-Bevacizumab Antibodies
- Anti-Brentuximab Antibodies
- Anti-Cemiplimab Antibodies
- Anti-Certolizumab Antibodies
- Anti-Cetuximab Antibodies
- Anti-Daratumumab Antibodies
- Anti-Denosumab Antibodies
- Anti-Dupilumab Antibodies
- Anti-Durvalumab Antibodies
- Anti-Eculizumab Antibodies
- Anti-Etanercept Antibodies
- Anti-Evolocumab Antibodies
- Anti-Golimumab Antibodies
- Anti-Infliximab Antibodies
- Anti-Ipilimumab Antibodies
- Anti-Natalizumab Antibodies
- Anti-Nivolumab Antibodies
- Anti-Obinutuzumab Antibodies
- Anti-Ocrelizumab Antibodies
- Anti-Omalizumab Antibodies
- Anti-Palivizumab Antibodies
- Anti-Panitumumab Antibodies
- Anti-Pembrolizumab Antibodies
- Anti-Pertuzumab Antibodies
- Anti-Ranibizumab Antibodies
- Anti-Rituximab Antibodies
- Anti-Secukinumab Antibodies
- Anti-Tocilizumab Antibodies
- Anti-Trastuzumab Antibodies
- Anti-Ustekinumab Antibodies
- Anti-Vedolizumab Antibodies
-
Our Anti-Biotherapeutic Antibodies
-
Anti-idiotypic Antibodies
s
s
s
Antibodies for bioanalysis and drug monitoring of golimumab and biosimilars
Develop highly selective and sensitive PK and ADA assays for the monoclonal antibody drug golimumab (Simponi) using our range of ready-made anti-idiotypic antibodies.
- Highly specific to golimumab or the golimumab-TNFα complex
- Fully human surrogate positive control or calibrator
- Sequence defined, well characterized reagents with secure supply
- Recombinant production, stringent quality control for batch to batch consistency
- Non-animal-derived antibodies, reducing the use of animals in science
Table 1. Antibodies Specific to Golimumab.
Specificity |
Catalog Number |
Clone |
Format |
Affinity* KD, nM |
Assay Recommendation |
Product Details |
|---|---|---|---|---|---|---|
|
Golimumab Inhibitory Type 1 |
HCA240 |
AbD20710 |
Fab-FH1 |
2.3 |
PK bridging ELISA |
|
|
HCA241 |
AbD20692_hIgG1 |
Human IgG1 |
2.8 |
PK bridging ELISA ADA control |
||
|
HCA286 |
AbD254292 |
Fab-FH1 |
1.0 |
PK bridging ELISA |
||
|
HCA287 |
AbD25418_hIgG12 |
Human IgG1 HRP |
0.1 |
PK bridging ELISA ADA control |
||
|
Golimumab & Golimumab-TNFα Non-Inhibitory Type 2 |
HCA242 |
AbD20897 |
Fab-FH1 |
7 |
PK bridging ELISA |
|
|
HCA243 |
AbD20897_hIgG1 |
Human IgG1 |
7 |
PK bridging ELISA |
||
|
HCA289 |
AbD254552 |
Fab-FH1 |
0.9 |
PK bridging ELISA |
||
|
HCA290 |
AbD25451_hIgG12 |
Human IgG1 HRP |
0.4 |
PK bridging ELISA |
||
|
Golimumab-TNFα Complex Specific Type 3 |
HCA274 |
AbD257052 |
Fab-FH1 |
6 |
PK ELISA antigen capture format |
|
|
HCA245 |
AbD20893_hIgG1 |
Human IgG1 |
53 |
PK ELISA antigen capture format |
* Affinity measured in the monovalent Fab format
1 Monovalent Fab antibody DYKDDDDK- and His-6-tags
2 Affinity matured antibodies: HCA286 is derived from AbD20710 (HCA240); HCA287 is derived from AbD20692 (HCA241); HCA289 and HCA290 are derived from AbD20897 (HCA242); HCA274 is derived from AbD20893 (HCA245)
Anti-Golimumab Inhibitory Antibodies (Type 1)
Type 1 anti-golimumab antibodies inhibit the binding of the drug golimumab to its target, tumor necrosis factor alpha (TNFα). They are ideal for development of a pharmacokinetic (PK) bridging ELISA to measure free drug. In fully human IgG1 format they are suitable as a surrogate positive control or calibrator for an anti-drug antibody (ADA) assay.
Anti-Golimumab Non-Inhibitory Antibodies (Type 2)
The type 2 anti-golimumab antibodies bind to the golimumab idiotope, but do not inhibit the binding of golimumab to TNFα. They can therefore be used in a PK bridging ELISA to measure total drug - free, partially bound and fully bound.
Anti-Golimumab-TNFα Complex Specific Antibody (Type 3)
The Type 3 antibodies specifically recognize the drug-target complex, detecting golimumab only when it is bound to TNFα. They are suitable as a detection antibody in a PK antigen capture assay.
ELISA Protocols to Get You Started
PK Bridging ELISA
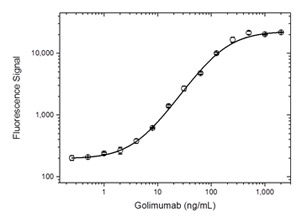
Fig. 1. Golimumab PK bridging ELISA using antibodies HCA286 and HCA287P.
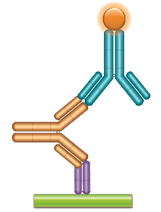
Schematic image of PK bridging ELISA measuring free drug. Anti-idiotypic capture antibody, Fab format (purple), monoclonal antibody drug (gold), anti-idiotypic detection antibody, Ig format (blue), labeled with HRP.
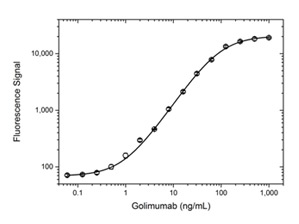
Fig. 2. Golimumab PK bridging ELISA using antibodies HCA289 and HCA290P.
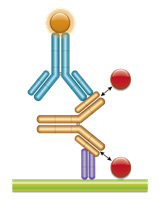
Schematic image of PK bridging ELISA measuring total drug. Type 2 anti-idiotypic capture antibody, Fab format (purple), monoclonal antibody drug (gold), drug target (red), type 2 anti-idiotypic detection antibody, Ig format (blue), labeled with HRP.
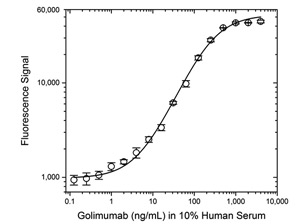
Fig. 3. Golimumab PK antigen capture ELISA using antibody HCA274.
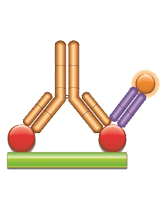
Schematic image of PK antigen capture ELISA. Drug target (red), monoclonal antibody drug (gold), drug-target complex detection antibody, monovalent Fab format (purple), labeled with HRP.
ADA Bridging ELISA
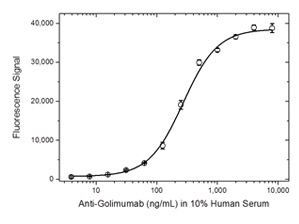
Fig. 4. Golimumab ADA bridging ELISA using antibody HCA287.
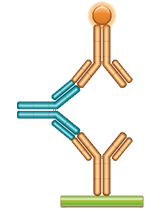
Schematic image of ADA bridging assay. Monoclonal antibody drug as capture antibody and detection antibody labeled with HRP (gold), fully human anti-idiotypic antibody, Ig format (blue).
Related Products
Resources
Licensed Use: For in vitro research purposes and for commercial applications for the provision of in vitro testing services to support preclinical and clinical drug development. Any re-sale in any form or any other commercial application needs a written agreement with Bio-Rad.