Anti-Infliximab Antibodies
-
Bioanalytical Antibodies
-
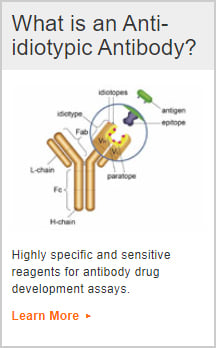
Anti-idiotypic Antibodies
-
Our Anti-Biotherapeutic Antibodies
- Anti-Abatacept Antibodies
- Anti-Adalimumab Antibodies
- Anti-Alemtuzumab Antibodies
- Anti-Atezolizumab Antibodies
- Anti-Avelumab Antibodies
- Anti-Belatacept Antibodies
- Anti-Bevacizumab Antibodies
- Anti-Brentuximab Antibodies
- Anti-Cemiplimab Antibodies
- Anti-Certolizumab Antibodies
- Anti-Cetuximab Antibodies
- Anti-Daratumumab Antibodies
- Anti-Denosumab Antibodies
- Anti-Dupilumab Antibodies
- Anti-Durvalumab Antibodies
- Anti-Eculizumab Antibodies
- Anti-Etanercept Antibodies
- Anti-Evolocumab Antibodies
- Anti-Golimumab Antibodies
- Anti-Infliximab Antibodies
- Anti-Ipilimumab Antibodies
- Anti-Natalizumab Antibodies
- Anti-Nivolumab Antibodies
- Anti-Obinutuzumab Antibodies
- Anti-Ocrelizumab Antibodies
- Anti-Omalizumab Antibodies
- Anti-Palivizumab Antibodies
- Anti-Panitumumab Antibodies
- Anti-Pembrolizumab Antibodies
- Anti-Pertuzumab Antibodies
- Anti-Ranibizumab Antibodies
- Anti-Rituximab Antibodies
- Anti-Secukinumab Antibodies
- Anti-Tocilizumab Antibodies
- Anti-Trastuzumab Antibodies
- Anti-Ustekinumab Antibodies
- Anti-Vedolizumab Antibodies
-
Our Anti-Biotherapeutic Antibodies
-
Anti-idiotypic Antibodies
s
s
s
Antibodies for bioanalysis and drug monitoring of infliximab and biosimilars
Develop highly selective and sensitive PK and ADA assays for infliximab (Remicade) using our range of ready-made anti-idiotypic antibodies.
- Inhibitory and non-inhibitory antibodies specific to infliximab
- Fully human surrogate positive control or calibrator
- Sequence defined, well characterized reagents with secure supply
- Recombinant production, stringent quality control for batch to batch consistency
- Non-animal-derived antibodies, reducing the use of animals in science
Table 1. Antibodies Specific to Infliximab.
Specificity |
Catalog Number |
Clone |
Format |
Affinity* KD, nM |
Assay Recommendation |
Product Details |
|---|---|---|---|---|---|---|
|
Infliximab Inhibitory Type 1 |
HCA212 |
AbD17837 |
Fab-V5Sx21 |
0.9 |
PK bridging ELISA |
|
|
HCA213 |
AbD17841_hIgG1 |
hIgG1 HRP |
1.8 |
PK bridging ELISA ADA control |
||
|
HCA233 |
AbD20436_hIgG1 |
hIgG1 |
0.12 |
PK bridging ELISA ADA control |
||
|
HCA234 |
AbD20436 |
Fab-FH2 |
0.12 |
PK bridging ELISA |
||
|
Infliximab & Infliximab-TNFα Non-Inhibitory Type 2 |
HCA214 |
AbD19376 |
Fab-FH2 |
0.7 |
PK bridging ELISA |
|
|
HCA215 |
AbD19370_hIgG1 |
hIgG1 |
1.4 |
PK bridging ELISA |
||
|
HCA216 |
AbD19376_hIgG1 |
hIgG1 HRP |
0.7 |
PK bridging ELISA |
||
|
Infliximab / TNFα Complex Specific Type 3 |
TZA009 |
AbD18391ad |
Fab-F-Spy2-H |
16
|
PK ELISA antigen capture format |
|
|
TZA009P |
AbD18391pap |
Fab2-FH-X22-HRP |
16
|
PK ELISA antigen capture format |
* Affinity measured in the monovalent Fab format
1 Monovalent Fab antibody, V5- and StrepX-StrepX- tags
2 Monovalent Fab antibody DYKDDDDK- and His-6-tags
Anti-Infliximab Inhibitory Antibodies (Type 1)
Type 1 anti-infliximab antibodies inhibit the binding of the drug infliximab to its target, tumor necrosis factor alpha, TNFα. They are ideal for development of a pharmacokinetic (PK) bridging ELISA to measure free drug. In fully human IgG1 format they are suitable as a surrogate positive control or calibrator for an anti-drug antibody (ADA) assay.
Anti-Infliximab Non-Inhibitory Antibodies (Type 2)
Type 2 anti-infliximab antibodies bind to the infliximab idiotope, but do not inhibit the binding of infliximab to TNFα. They are ideal for development of a PK bridging ELISA to measure total drug - free, partially bound and fully bound.
Anti-Infliximab TNFα Complex Specific Antibody (Type 3)
The Type 3 antibodies specifically recognize the drug-target complex, detecting infliximab only when it is bound to TNFα. They are suitable as a detection antibody in a PK antigen capture assay.
ELISA Protocols to Get You Started
PK Bridging ELISAs
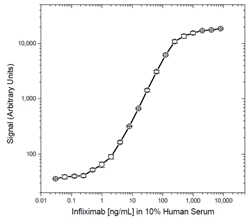
Fig. 1. Infliximab PK bridging ELISA using antibodies HCA212 and HCA213P.
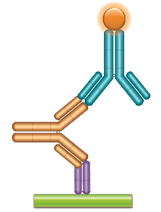
Schematic image of PK bridging ELISA detecting free drug. Anti-idiotypic capture antibody, Fab format (purple), monoclonal antibody drug (gold), anti-idiotypic detection antibody, Ig format (blue), labeled with HRP.
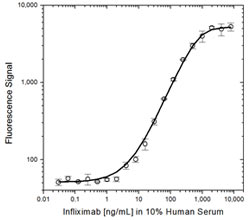
Fig. 2. Infliximab PK ELISA bridging format using antibodies HCA214 and HCA216P.
Schematic image of PK bridging ELISA detecting total drug. Anti-idiotypic capture antibody, Fab format (purple), monoclonal antibody drug (gold), anti-idiotypic detection antibody, Ig format (blue), labeled with HRP.
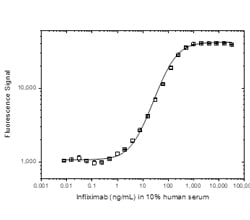
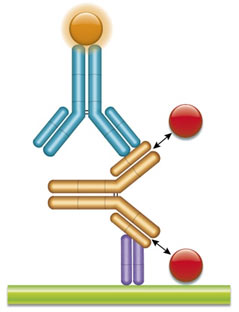
Fig. 3. Infliximab PK antigen capture ELISA using TZA009P.
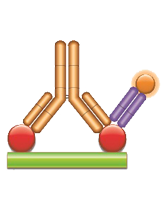
Schematic image of PK antigen capture ELISA. Drug target (red), monoclonal antibody drug (gold), drug-target complex detection antibody, monovalent Fab format (purple), labeled with HRP.
ADA Bridging ELISA
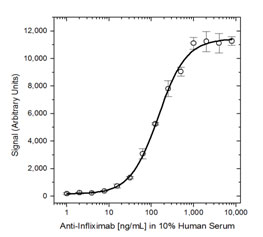
Fig. 4. ADA bridging ELISA using antibody HCA213.
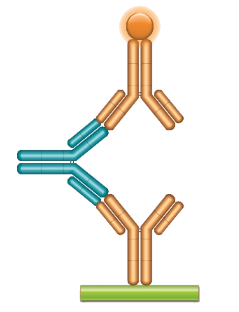
Schematic image of ADA bridging assay. Monoclonal antibody drug as capture antibody and detection antibody labeled with HRP (gold), fully human anti-idiotypic antibody, Ig format (blue).
Related Products
Resources
Licensed Use: For in vitro research purposes and for commercial applications for the provision of in vitro testing services to support preclinical and clinical drug development. Any re-sale in any form or any other commercial application needs a written agreement with Bio-Rad.