Unlabeled Primary Antibodies with Fluorescently-Labeled Secondary Products, Resources, and Tools

- On This Page
- Products
- Protocol
- Resources
To get the best from your indirect staining, we’ve collated information on how to choose the ideal secondary antibody alongside useful tools for fluorophore selection, including our Panel Builder and Spectraviewer.
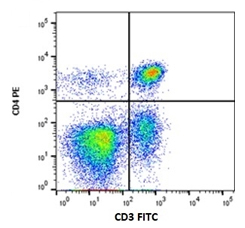
Fig.1. RPE conjugated Rat anti-Dog CD4 (MCA1038PE) and Mouse anti-Dog CD3 (MCA1774GA) detected with Goat anti-Mouse IgG:FITC (STAR117F).
Products
As specialists in the flow cytometry field, our antibody portfolio includes a selection of secondary antibodies tested in flow cytometry. Feel free to filter the table below, using our target species button or the individual filters within the table, to find exactly what you are looking for.
As well as our range of secondary antibodies, we offer an extensive selection of flow cytometry tested primary antibodies including our new StarBright™ Dyes, conjugated antibodies, and a large selection of additional products/consumables to help you achieve successful experiments. These include:
- Primary antibodies
- StarBright Dye conjugated primary antibodies
- Labeling kits to conjugate antibodies
- Flow cytometry reagents
- Antibody cocktails
- Apoptosis detection kits
We provide a large, comprehensive range of useful educational flow cytometry resources that teach you how to overcome common challenges when building flow cytometry panels, including experimental data demonstrating the benefits and ease of use of StarBright Dyes and an article on Using Secondary Reagents in Flow Cytometry with Confidence.
Protocol
Indirect Immunofluorescence Staining of Cells and Blood for Flow Cytometry
This technique is applicable when using unconjugated or biotin-conjugated monoclonal and polyclonal antibodies recognizing cell surface antigens. A conjugated secondary reagent must be used to visualize the primary antibody, for example streptavidin in the case of biotin.
This method provides a general procedure for use with the majority of Bio-Rad reagents. In some cases specific recommendations are provided on product datasheets, and these methods should always be used in conjunction with the product and batch specific information provided with each vial. A certain level of technical skill and immunological knowledge is required for the successful design and implementation of these techniques; these are guidelines only and may need to be adjusted for particular applications. Specific methodology for blood appears in [ ] brackets.
Reagents
- Phosphate buffered saline (BUF036A) containing 1% bovine serum albumin (PBS/BSA).
- Erythrolyse red blood cell lysing buffer (BUF04).
- Anticoagulant (Note: for basic staining any appropriate anticoagulant, such as heparin, EDTA, or acid citrate dextrose, may be used. In some instances specific anticoagulants may be required)
- Optional: 0.5% (w/v) paraformaldehyde in PBS (Note: dissolve on heated stirrer and cool before use)
Method
-
Prepare cells appropriately; refer to protocol FC1. 'Preparation of cells for flow cytometry' for further information. Adjust the cell suspension to a concentration of 1 x 107 cells/ml with cold (4oC) PBS/BSA buffer.
[Whole blood samples may be used undiluted unless the cell count is high, e.g. as in leukemia. Use appropriate anticoagulant].
- Aliquot 100 μl of the cell suspension [or whole blood] into as many test tubes as required.
- Add primary antibody at the vendor-recommended dilution. Mix well and incubate at 4oC for at least 30 min.
-
Wash cells with 2 ml of cold (4oC) PBS/BSA, centrifuge at 300-400 g for 5 min, and discard the supernatant.
[To the blood suspension add 2 ml freshly prepared red cell lysis buffer and mix well. Incubate for 10 min at room temperature. Centrifuge at 300-400 g for 5 min and discard the supernatant. Wash with 2 ml of PBS/BSA, centrifuge at 300-400 g for 5 min, and discard the supernatant].
- Add an appropriate secondary reagent at the vendor-recommended dilution. Mix well and incubate at 4oC for at least 30 min, avoiding direct light.
- Centrifuge at 300-400 g for 5 min at room temperature and discard the supernatant.
- Re-suspend cells in 200 μl of cold (4oC) PBS or with 200 μl of 0.5% paraformaldehyde in PBS if required.
- Acquire data by flow cytometry. Analyze fixed cells within 24 hours.
Notes
-
To avoid nonspecific binding, you also need to block Fc receptors on cell types such as spleen cells, with FcR blocking reagents e.g. Bio-Rad’s Mouse Seroblock reagent (BUF041).
-
Appropriate controls should always be carried out, for flow cytometry the following should be considered for inclusion;
-
Isotype controls used to determine if the staining is specific.
-
Unstained cells should always be included in the experimental set up to monitor autofluorescence.
-
- For all multicolor flow cytometry experiments it is advisable to include compensation controls and fluorescence minus one (FMO) controls, which assist with identifying gating boundaries.

How to Use Secondary Antibodies — Frequently Asked Questions
Resources
Secondary Antibody Troubleshooting FAQs
Improve your experimental results when using secondary antibodies with our troubleshooting guidance for when your experiments go wrong.
Separated into general and application-specific FAQs to enable quick and easy access to the information that is relevant to you.

Secondary Antibodies - Optimize your Detection System
Secondary Antibody Selection
Using the knowledge from the “Experimental design — how-to reference guide” section and your desired application, follow the steps below to discover your ideal secondary antibody.
Select:
- Target species
- Host
- Class and chain (found in the specificity filter)
- Isotype
- Format
- Check before you select — does your antibody need to be cross-adsorbed?
Use the filters to systemically select your requirements and discover the best secondary antibody for your experiment, then download the results.
Secondary Antibodies Range
| Description | Target | Host | Specificity | Format | Citations | Isotype | Code |
|---|
- When using more than one secondary antibody ensure that they don’t cross react
- When working with some immune tissues or cells that contain a lot of Fc receptors, it helps to choose a F(ab) or F(ab’)2 fragment to eliminate non-specific binding. Alternatively, block Fc receptors via an absorption step, using purified IgG from the host species of your secondary antibody or serum from target cells
- View our IHC tips when using a secondary antibody
Any questions? Let us help, contact our technical support team for advice
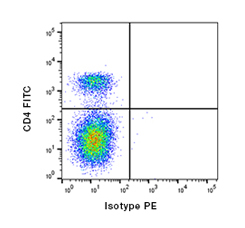
Fig.2. Staining of bovine peripheral blood T lymphocytes using direct and indirect staining. A, FITC conjugated Mouse anti-Bovine CD4 (MCA1653F) and Mouse IgG1 Isotype Control (MCA928) detected with PE conjugated Anti-Mouse IgG1 (STAR132PE).
Using Secondary Reagents in Flow Cytometry with Confidence
Flow cytometry predominantly uses direct staining with conjugated primary antibodies to detect markers of interest, especially when multiplexing for immunophenotyping, however, not all antibodies are available with a fluorescent marker or one that is compatible with other fluorophores or fluorescent proteins. For example, if an antibody is only available in an unconjugated version, or only a FITC conjugate is available and staining is required in a GFP positive sample.
Often the solution to this problem is to conjugate the unlabeled primary antibody to a fluorophore of your choice, using a LYNX or ReadiLink Kit. However, there may be occasions when this approach is not practical; there may be a limited amount of antibody available, the antibody may be impure or contain a carrier protein, critical amino acids at the antigen binding site may be disrupted by conjugation, or in the case of screening hybridomas there may simply be too many antibodies. In this situation, indirect staining using a secondary antibody is invaluable for detecting your marker.
Choosing Fluorophores for Flow Cytometry

Fluorophores and Viability Dyes for Flow Cytometry
Reference Postcard
Flow cytometry generally requires bright fluorophores that are excitable by one laser with a narrow emission wavelength to allow multicolor panel building. Below is a handy guide to the range of fluorophores Bio-Rad offers, including our new StarBright™ Dyes, that are suitable for flow cytometry, grouped by the laser used to excite them.
Additional fluorophore information can be found on specific webpages organized by the laser that excites them. This includes the maximal emission wavelength, relative brightness, ZE5 Cell Analyzer filter used for detection, similar or incompatible fluorophores, and our antibodies available conjugated to them, helping you select the right fluorophore for your experiment.
For more information on which fluorophores are the most suitable for your experiment, try our fluorescent spectraviewer or download our poster showing common fluorophores used in flow cytometry.
Fluorophores excited by the 355 nm (Ultraviolet) Laser
- StarBright UltraViolet 400
- StarBright UltraViolet 445
- StarBright UltraViolet 510
- StarBright UltraViolet 575
- StarBright UltraViolet 605
- StarBright UltraViolet 665
- StarBright UltraViolet 740
-
StarBright UltraViolet 795
Fluorophores Excited by the 405 nm (Violet) Laser
- Pacific Blue
- StarBright Violet 440
- StarBright Violet 475
- StarBright Violet 515
- Amethyst Orange
- StarBright Violet 570
- StarBright Violet 610
- StarBright Violet 670
- StarBright Violet 710
- StarBright Violet 760
-
StarBright Violet 790
Fluorophores Excited by the 488 nm (Blue) Laser
- FITC
- Alexa Fluor 488
- PE
- PE-Alexa Fluor647
- PE-Cy5
- PE-Cy5.5
- PE-Alexa Fluor750
- PE-Cy7
- PerCP
- StarBright Blue 580
- StarBright Blue 615
- StarBright Blue 675
- StarBright Blue 700
- StarBright Blue 765
-
StarBright Blue 810
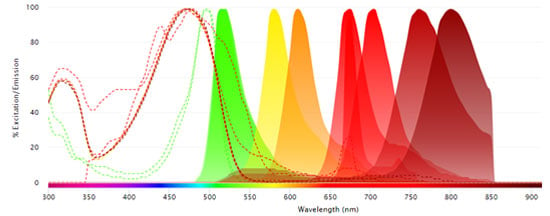
Fluorophores Excited by the 561 nm (Yellow) Laser
- PE
- PE-Alexa Fluor647
- PE-Cy5
- PE-Cy5.5
- PE-Alexa Fluor750
- PE-Cy7
- StarBright Yellow 575
- StarBright Yellow 605
- StarBright Yellow 665
- StarBright Yellow 720
-
StarBright Yellow 800
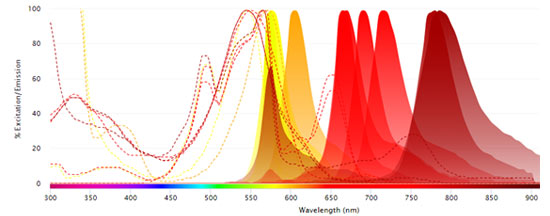
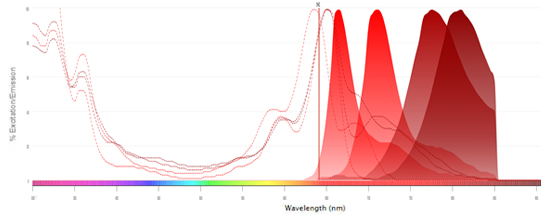
Fluorophores Excited by the 640 nm (Red) Laser
- Alexa Fluor 647
- APC
- Alexa Fluor 700
- StarBright Red 670
- StarBright Red 715
- StarBright Red 775
-
StarBright Red 815
Antigen Density of Common Human and Murine Markers
Quick Guide
Antigen Density
Tutorials for building multicolor flow cytometry panels always highlight the importance of antigen density — but why is it so important?
The number of antigens, or target molecules that a cell carries directly correlates to the intensity of the positive population and will determine the optimal fluorophore you should use for each marker. As a general rule you should pair bright fluorophores (e.g. PE) with low expressing markers and dimmer fluorophores (e.g. Pacific Blue) with highly expressed markers. Careful choice of fluorophores will help with the resolution of your cell populations. It is possible for bright fluorophores to be paired with highly expressed antigens, but better to avoid pairing dim fluorophores with low abundance antigens.
The guide provides advice on how to measure antigen density and lists the antigen density of some common markers.

Essentials for Multicolor Panel Building Poster, incl. tips on antibody titration.
Antibody Titration in Flow Cytometry
To optimize staining in flow cytometry antibody titration is recommended. While antibodies will bind to high affinity targets on a cell, if they are present in excess they will also bind to low affinity targets. This results in an increase in background fluorescence and consequently a reduction in your ability to resolve populations, especially if there are subtle differences. Furthermore if the antibody concentration is too high, it may result in a false negative prozone effect.
It is therefore important to use the antibodies at the right concentration. Although antibodies are sold with a recommended dilution and it is a good starting point, it may not be optimal for your cell type or protocol, therefore titration is an essential step to optimize your staining.
Basic Multicolor Panel Design Rules
Before you build your panels it is critically important to have as clear an idea as possible of what is required to identify your population of interest. For example, are you looking at one cell type or is it a subset, are you looking at an activation marker or a change in cell frequency, are the markers co-expressed or do you even know the expression pattern?
Ideally, when building multicolor panels, it is best to separate fluorophore excitation across lasers, and where possible, the emission across the detectors. This will minimize the amount of spillover and therefore compensation you will need to do. It will also reduce the effect that fluorescence spread will have on your data. However, as you increase the number of fluorophores in your panel, this will not always be possible. Therefore other considerations need to be included in your design.
Webinar: Multicolor Panel Building in Flow Cytometry
Presented by: Dr Sebastian Hedlund, Flow Cytometry Specialist at Bio-Rad Laboratories
Multicolor flow cytometry is the analysis of multiple fluorescent parameters in one sample. Building large flow cytometry panels can be daunting because each additional fluorophore you add to your panel has the potential to influence another fluorophore.
Join our 45 minute webinar to learn which fluorophores are compatible with each other, how they interact and can affect your staining and how to obtain optimal resolution of signal. Furthermore, we will show you how dump channels, fluorophore brightness, antigen density, marker expression patterns and instrument configuration can all contribute to improving your panel design.
This webinar is recommended for both novices and researchers with some experience of flow cytometry.
Speaker Biography
 Sebastian obtained his degree from the Institute of Technology, Linköping University, Sweden, followed by an MSc in Medical Biology from the same institute. He then moved to Freiburg, Germany where he obtained his PhD in Immunology and Molecular Biology at the Max Planck Institute of Immunobiology and Epigenetics.
Sebastian obtained his degree from the Institute of Technology, Linköping University, Sweden, followed by an MSc in Medical Biology from the same institute. He then moved to Freiburg, Germany where he obtained his PhD in Immunology and Molecular Biology at the Max Planck Institute of Immunobiology and Epigenetics.
The focus of his thesis was the role of microRNA in innate immunity and immunopathology. He then left academia and started work as a dedicated flow cytometry specialist, before joining Bio-Rad in 2016 as the flow cytometry specialist for Scandinavia, Eastern Europe, Israel, and South Africa.
Multicolor Panel Builder
Build multicolor flow cytometry panels in just a few simple steps
- Start your design by first selecting your instrument from the drop down menu, or create a customized instrument, to build a personalized panel
- Select the target and species you want to identify. Keep adding until you have all the markers you want
- Choose antibodies by clone, isotype and format. If there is not an antibody available in the format you require, select the fluorophore as a placeholder and continue
- Include a viability dye or dump channel for more complexity
The ability to select antigen density and fluorophores based on brightness, combined with automatic exclusion of incompatible fluorophores, will help you build larger panels with confidence.
Interactive Flow Cytometry, Spectral Analyzer, Microscopy, and Western Blot Fluorescent Spectraviewer
Find the right fluorophore for your application using our new interactive, fluorescent spectraviewer with hundreds of fluorophores to choose from.
Bio-Rad’s new spectraviewer is the only spectraviewer to allow a multi-laser view and support three applications in a single tool.
- Supports flow cytometry, fluorescence microscopy, and western blotting
- Displays the excitation and emission profiles of the fluorophores available to you, essential when building multicolor panels for flow cytometry
- Allows full customization: select any fluorophore, laser, and filter combination
- Provides more information than any other spectraviewer: the traditional view, showing the fluorescence spectra, is complemented by the multi-laser view visualizing the spillover and cross-laser excitation and emission
- Saves time with the pre-loaded Bio-Rad instrument settings
The ability to view in a multi-laser format allows you to check fluorophore compatibility, including potential issues due to cross-laser excitation, and predict compensation.
Bio-Rad has over 4,000 antibodies validated for flow cytometry as well as helpful resources to guide you through your flow cytometry experiments. These resources as well as listings of antibodies, kits, and controls can be accessed from our dedicated flow cytometry page.






