Isotype Controls for Flow Cytometry

- On This Page
- Determine Specific Binding
- What are Isotype Controls?
- Determine Background Staining
- Other Controls
- Flow Negative Isotype Controls
Determine Specific Binding
The debate about whether, when and how to use isotype controls appears to be controversial to researchers (Herzenberg L et al., Maecker HT and Trotter J.). You may not wish to use isotype controls, but if you do this article will give you an overview of what an isotype control is, when and how to use it. Remember they are only one of a number of appropriate controls that will improve you flow cytometry data. For more information on controls in flow cytometry, we have a dedicated page, with a more detailed overview of controls in flow cytometry.
What are Isotype Controls?
Unwanted background cell staining in flow cytometry can be a problem, especially when detecting novel or rare populations and when building panels containing multiple fluorophores. An isotype control is an antibody raised against an antigen not present on the cell type being analyzed (e.g. KLH or DNP) and has been specifically developed to determine the level of background surface staining.
An isotype control will:
- Determine the non-specific binding of an antibody to Fc receptors found on monocytes, macrophages, dendritic and B cells
- Ensure the observed staining is due to specific binding rather than an artifact
- Reveal other non-specific binding of the antibody or fluorophores to cellular components (e.g. RPE and FITC, Takizawa et al, 1993, Hulspas et al. 2009)
How an Isotype Control can help Determine Background Staining
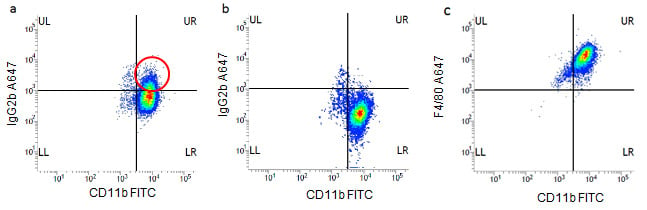
Fig 1. J774 macrophages were stained for 30 minutes at 4oC in PBS w/v1%BSA with CD11b FITC (MCA74F) and IgG2b Alexa Fluor®647 (MCA6006A647) a). In the absence of Fc block or b). In the presence of mouse Fc block (mouse Seroblock FCR, BUF041A). In Fig1a there is a significant population of cells positive (circled) with the A647 isotype control showing the Fc binding which disappears when Fc block is included, Fig1b. Fig1c shows specific F4/80 A647 (MCA497A647) staining. All data shown was gated on the 7-AAD negative, live population with the use of doublet discrimination.
The role of isotype controls in determining background staining can be observed in Figure 1a where no Fc block has been used (false positive cells circled red) compared to Figure 1b where Fc block has been added. The specific F4/80 staining can be clearly seen in figure 1c.
The most appropriate isotype control matches:
- The host species
- Ig subclass
- Fluorophore of the primary antibody
If you are using a mouse IgG1 monoclonal antibody that is conjugated to FITC, you should select a mouse IgG1 isotype control conjugated to FITC.
As the fluorophore conjugation to the antibody (known as the F/P ratio) can vary between suppliers, it is best to purchase the isotype from the same supplier as the primary. It is also advisable to use them at the same concentration as the primary antibody. If you want to calculate the F/P ratio of your antibodies we explain how in four easy steps.
Isotype controls have been optimized for surface staining. Intracellular staining can be affected by binding of both antibody and fluorophore to intracellular components, therefore choice of fluorophore and extra controls may be necessary. Remember isotype controls should not be used to determine compensation levels or the negative population.
Non-specific antibody binding can be reduced by:
- Blocking Fc receptors
- Adding excess protein such as BSA to your buffer
- Titration of your antibody
- Gating out dead cells using live/dead marker
To see our range of flow cytometry isotype controls with information on how and when to use them download our isotype controls brochure. Alternatively you can use our handy search table located at the bottom of the page to find the right isotype control for your experiment.
Other Controls
As described, isotype controls are used to determine if the staining is specific. Apart from isotype controls, unstained cells should always be included in the experimental set-up to monitor autofluorescence. Additionally, for all multi-color flow cytometry experiments it is advisable to include compensation controls and Fluorescence Minus One (FMO) controls, which assist with identifying gating boundaries. We discuss other controls on our flow cytometry controls page.
Flow Negative Isotype Controls
| Description | Target | Format | Clone | Applications | Citations | Code |
|---|
The references below will provide extra information if you are interested in finding out more about isotype controls and their use in flow cytometry.
-
Herzenberg et al. Nat Imm 2006
-
Maeker HT and Trotter J. Cytometry Part A 2006
-
Takizawa F et al, 1993. J Immunol Methods
-
Hulspas et al. 2009, Cytometry B Clin Cytom






