Fluorescently-labeled Primary Antibodies, Resources, and Tools

- On This Page
- Products
- Protocol
- Resources
Alongside tips for direct staining, discover the ideal fluorophore for your experiment. Our handy fluorophore guide, Panel Builder, and Spectraviewer tools make fluorophore choice simple.
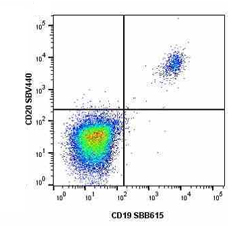
Fig.1. Staining of human lymphocytes with StarBright Violet 440 conjugated Mouse anti Human CD20 (MCA1710SBV440) and StarBright Blue 615 conjugated Mouse anti Human CD19 (MCA1940SBB615).
Products
Flow Cytometry Antibodies
As specialists in the flow cytometry field, our antibody portfolio includes an extensive selection of primary antibodies tested in flow cytometry, including our new StarBright™ Dye conjugated antibodies.
Feel free to filter the table below, using our target species button or the individual filters within the table, to find exactly what you are looking for.
As well as our range of flow cytometry tested antibodies, we offer a large selection of additional products/consumables to help you achieve successful experiments. These include:
Our Flow Cytometry Antibody Range
| Description | Target | Format | Clone | Applications | Citations | Code |
|---|

StarBright Dyes
StarBright Dyes have been designed to be stable with superior brightness, narrow excitation and emission characteristics, and the flexibility to be included in new and existing experiments. Improved resolution of specific cell populations and minimized spillover and spreading allow you to build bigger, better panels with ease.
Conjugated to highly cited immunology antibodies, StarBright Dyes are a great addition to Bio-Rad’s range of fluorescent dyes available for ultraviolet, violet, blue, and yellow lasers suitable for use on all cell sorters, cell analyzers, and spectral instruments.
Protocol
Direct Immunofluorescence Staining of Cells and Blood
Applicable where the fluorophore is directly linked to the primary antibody e.g. RPE, FITC, and Alexa Fluor® conjugates. RPE conjugates should always be handled in the dark.
This method provides a general procedure for use with the majority of Bio-Rad reagents. In some cases specific recommendations are provided on product datasheets, and these methods should always be used in conjunction with the product and batch specific information provided with each vial. A certain level of technical skill and immunological knowledge is required for the successful design and implementation of these techniques; these are guidelines only and may need to be adjusted for particular applications. Specific methodology for blood appears in [ ] brackets.
Reagents
- Phosphate buffered saline (BUF036A) containing 1% bovine serum albumin (PBS/BSA).
- Erythrolyse red blood cell lysing buffer (BUF04).
- Anticoagulant (Note: for basic staining any appropriate anticoagulant, such as heparin, EDTA, or acid citrate dextrose, may be used. In some instances specific anticoagulants may be required)
- Optional: 0.5% (w/v) paraformaldehyde in PBS (Note: dissolve on heated stirrer and cool before use)
Method
-
Prepare cells appropriately; refer to protocol FC1. 'Preparation of cells for flow cytometry' for further information. Adjust the cell suspension to a concentration of 1 x 107 cells/ml with cold (4oC) PBS/BSA buffer.
[Whole blood samples may be used undiluted unless the cell count is high, e.g. as in leukemia. Use appropriate anticoagulants].
- Aliquot 100 μl of the cell suspension [or whole blood] into as many test tubes as required.
-
Add antibody at the vendor-recommended dilution. Mix well and incubate at 4oC for at least
30 min, avoiding direct light. -
Wash cells with 2 ml cold (4oC) PBS/BSA, centrifuge at 300-400 g for 5 min at 4oC, and discard the resulting supernatant.
[To the blood suspension add 2 ml freshly prepared erythrolyse red cell lysing buffer and mix well. Incubate for 10 min at room temperature. Centrifuge at 300-400 g for 5 min at room temperature and discard the supernatant. Wash with 2 ml room temperature PBS/BSA, centrifuge at 300-400 g for 5 min and discard the supernatant. Proceed to step 5].
- Re-suspend cells in 200 μl cold (4oC) PBS or with 200 μl of 0.5% paraformaldehyde in PBS if required.
- Acquire data by flow cytometry. Analyze fixed cells within 24 hours.
Notes
-
The following should be considered when designing your flow cytometry experiments:
-
To avoid nonspecific binding, you also need to block Fc receptors on cell types such as spleen cells, with FcR blocking reagents e.g. Bio-Rad’s Mouse Seroblock reagent (BUF041).
-
Appropriate controls should always be carried out, for flow cytometry the following should be considered for inclusion;
-
Isotype controls used to determine if the staining is specific
-
Unstained cells should always be included in the experimental set-up to monitor autofluorescence.
-
-
-
For all multicolor flow cytometry experiments it is advisable to include compensation controls and fluorescence minus one (FMO) controls, which assist with identifying gating boundaries.
Resources
Choosing Fluorophores for Flow Cytometry

Fluorophores and Viability Dyes for Flow Cytometry
Reference Postcard
Flow cytometry generally requires bright fluorophores that are excitable by one laser with a narrow emission wavelength to allow multicolor panel building. Below is a handy guide to the range of fluorophores Bio-Rad offers, including our new StarBright Dyes, that are suitable for flow cytometry, grouped by the laser used to excite them.
Additional fluorophore information can be found on specific webpages organized by the laser that excites them. This includes the maximal emission wavelength, relative brightness, ZE5 Cell Analyzer filter used for detection, similar or incompatible fluorophores, and our antibodies available conjugated to them, helping you select the right fluorophore for your experiment.
For more information on which fluorophores are the most suitable for your experiment, try our fluorescence spectraviewer or download our poster showing common fluorophores used in flow cytometry.
Fluorophores excited by the 355 nm (Ultraviolet) Laser
- StarBright UltraViolet 400
- StarBright UltraViolet 445
- StarBright UltraViolet 510
- StarBright UltraViolet 575
- StarBright UltraViolet 605
- StarBright UltraViolet 665
- StarBright UltraViolet 740
-
StarBright UltraViolet 795
Fluorophores Excited by the 405 nm (Violet) Laser
- Pacific Blue
- StarBright Violet 440
- StarBright Violet 475
- StarBright Violet 515
- Amethyst Orange
- StarBright Violet 570
- StarBright Violet 610
- StarBright Violet 670
- StarBright Violet 710
- StarBright Violet 760
-
StarBright Violet 790
Fluorophores Excited by the 488 nm (Blue) Laser
- FITC
- Alexa Fluor 488
- PE
- PE-Alexa Fluor647
- PE-Cy5
- PE-Cy5.5
- PE-Alexa Fluor750
- PE-Cy7
- PerCP
- StarBright Blue 580
- StarBright Blue 615
- StarBright Blue 675
- StarBright Blue 700
- StarBright Blue 765
-
StarBright Blue 810
Fluorophores Excited by the 561 nm (Yellow) Laser
- PE
- PE-Alexa Fluor647
- PE-Cy5
- PE-Cy5.5
- PE-Alexa Fluor750
- PE-Cy7
- StarBright Yellow 575
- StarBright Yellow 605
- StarBright Yellow 665
- StarBright Yellow 720
-
StarBright Yellow 800
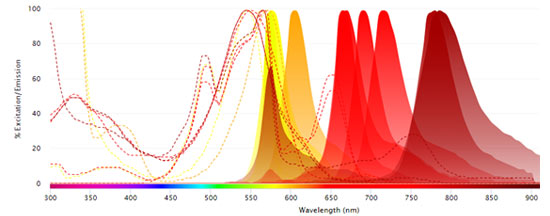
Fluorophores Excited by the 640 nm (Red) Laser
- Alexa Fluor 647
- APC
- Alexa Fluor 700
- StarBright Red 670
- StarBright Red 715
- StarBright Red 775
-
StarBright Red 815
Antigen Density of Common Human and Murine Markers
Quick Guide
Antigen Density
Tutorials for building multicolor flow cytometry panels always highlight the importance of antigen density — but why is it so important?
The number of antigens, or target molecules that a cell carries directly correlates to the intensity of the positive population and will determine the optimal fluorophore you should use for each marker. As a general rule you should pair bright fluorophores (e.g. PE) with low expressing markers and dimmer fluorophores (e.g. Pacific Blue) with highly expressed markers. Careful choice of fluorophores will help with the resolution of your cell populations. It is possible for bright fluorophores to be paired with highly expressed antigens, but better to avoid pairing dim fluorophores with low abundance antigens.
The guide provides advice on how to measure antigen density and lists the antigen density of some common markers.

Essentials for Multicolor Panel Building Poster, incl. tips on antibody titration.
Antibody Titration in Flow Cytometry
To optimize staining in flow cytometry antibody titration is recommended. While antibodies will bind to high affinity targets on a cell, if they are present in excess they will also bind to low affinity targets. This results in an increase in background fluorescence and consequently a reduction in your ability to resolve populations, especially if there are subtle differences. Furthermore, if the antibody concentration is too high, it may result in a false negative prozone effect.
It is therefore important to use the antibodies at the right concentration. Although antibodies are sold with a recommended dilution and it is a good starting point, it may not be optimal for your cell type or protocol, therefore titration is an essential step to optimize your staining.
Basic Multicolor Panel Design Rules
Before you build your panels it is critically important to have as clear an idea as possible of what is required to identify your population of interest. For example, are you looking at one cell type or is it a subset, are you looking at an activation marker or a change in cell frequency, are the markers co-expressed or do you even know the expression pattern?
Ideally, when building multicolor panels, it is best to separate fluorophore excitation across lasers, and where possible, the emission across the detectors. This will minimize the amount of spillover and therefore compensation you will need to do. It will also reduce the effect that fluorescence spread will have on your data. However, as you increase the number of fluorophores in your panel, this will not always be possible. Therefore other considerations need to be included in your design.
Webinar: Multicolor Panel Building in Flow Cytometry
Presented by: Dr Sebastian Hedlund, Flow Cytometry Specialist at Bio-Rad Laboratories
Multicolor flow cytometry is the analysis of multiple fluorescent parameters in one sample. Building large flow cytometry panels can be daunting because each additional fluorophore you add to your panel has the potential to influence another fluorophore.
Join our 45 minute webinar to learn which fluorophores are compatible with each other, how they interact and can affect your staining, and how to obtain optimal resolution of signal. Furthermore, we will show you how dump channels, fluorophore brightness, antigen density, marker expression patterns, and instrument configuration can all contribute to improving your panel design.
This webinar is recommended for both novices and researchers with some experience of flow cytometry.
Speaker Biography
 Sebastian obtained his degree from the Institute of Technology, Linköping University, Sweden, followed by an MSc in Medical Biology from the same institute. He then moved to Freiburg, Germany where he obtained his PhD in Immunology and Molecular Biology at the Max Planck Institute of Immunobiology and Epigenetics.
Sebastian obtained his degree from the Institute of Technology, Linköping University, Sweden, followed by an MSc in Medical Biology from the same institute. He then moved to Freiburg, Germany where he obtained his PhD in Immunology and Molecular Biology at the Max Planck Institute of Immunobiology and Epigenetics.
The focus of his thesis was the role of microRNA in innate immunity and immunopathology. He then left academia and started work as a dedicated flow cytometry specialist, before joining Bio-Rad in 2016 as the flow cytometry specialist for Scandinavia, Eastern Europe, Israel, and South Africa.
Multicolor Panel Builder
Build multicolor flow cytometry panels in just a few simple steps
- Start your design by first selecting your instrument from the drop down menu, or create a customized instrument, to build a personalized panel
- Select the target and species you want to identify. Keep adding until you have all the markers you want
- Choose antibodies by clone, isotype, and format. If there is not an antibody available in the format you require, select the fluorophore as a placeholder and continue
- Include a viability dye or dump channel for more complexity
The ability to select antigen density and fluorophores based on brightness, combined with automatic exclusion of incompatible fluorophores, will help you build larger panels with confidence.
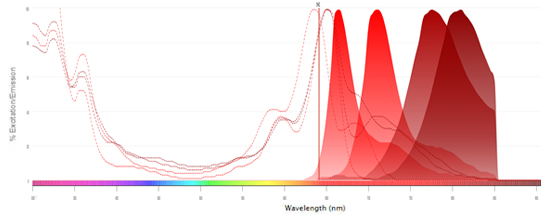
Interactive Flow Cytometry, Spectral Analyzer, Microscopy, and Western Blot Fluorescent Spectraviewer
Find the right fluorophore for your application using our new interactive, fluorescent spectraviewer with hundreds of fluorophores to choose from.
Bio-Rad’s new spectraviewer is the only spectraviewer to allow a multi-laser view and support three applications in a single tool.
- Supports flow cytometry, fluorescence microscopy, and western blotting
- Displays the excitation and emission profiles of the fluorophores available to you, essential when building multicolor panels for flow cytometry
- Allows full customization: select any fluorophore, laser, and filter combination
- Provides more information than any other spectraviewer: the traditional view, showing the fluorescence spectra, is complemented by the multi-laser view visualizing the spillover and cross-laser excitation and emission
- Saves time with the pre-loaded Bio-Rad instrument settings
The ability to view in a multi-laser format allows you to check fluorophore compatibility, including potential issues due to cross-laser excitation, and predict compensation.
Bio-Rad has over 4,000 antibodies validated for flow cytometry as well as helpful resources to guide you through your flow cytometry experiments. These resources as well as listings of antibodies, kits, and controls can be accessed from our dedicated flow cytometry page.





