Apoptosis: Induction Phase of Apoptosis
Induction Overview
Induction of
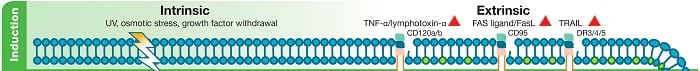
Fig. 1. Induction of apoptosis. One of two apoptotic pathways will be activated. Intrinsic apoptosis is activated by internal DNA damage within the cell. Potential causes of this DNA damage are UV light and reactive oxygen species. Extrinsic apoptosis is activated by binding of ligands to “death receptors”.
Intrinsic Pathway
The intrinsic pathway can be initiated by conditions such as a withdrawal of growth factor supplements from culture media, exposure to UV light or cell stresses (for instance osmotic or metabolic). These conditions ultimately lead to the damage of DNA cells. In the case of exposure to UV light, the adsorption of a UVB photon causes adjoining thymine base pairs to form pyridimine dimers. Growth factor withdrawal or other stressful conditions can lead to increased production of reactive oxygen species (ROS). ROS can damage DNA by several means including base modification, single strand breaks and crosslinking. DNA damage exerts a pro-apoptotic influence on members of the B-Cell Lymphoma 2 (Bcl-2) family which regulate the intrinsic apoptosis pathway.
Extrinsic Pathway
The extrinsic pathway is triggered by the binding of ligands to so called “death receptors” (DR), for instance CD95, DR3/4/5 as shown in Figure 1. The death receptors CD120a, CD95 and TRAIL receptors, called DR3, DR4, DR5 and DR6, are grouped under the TNF receptor superfamily. This family also includes TWEAK (TNF-like weak inducer of apoptosis) receptor (CD266 / Fn14) which interacts with its ligand TWEAK (CD255) inducing apoptosis in some tumor cell lines through pathways yet to be fully elucidated.
Upon ligand binding, most death receptors oligomerize and recruit adaptor proteins such as FADD and the pro-caspases -8 and -10. This assembly and recruitment occur as a result of the presence of protein interaction modules called the death domains. Death domains are 80–100 residue long motifs found on DRs and other molecules associated with apoptotic signaling. Individual death domains bind to one another through complementary electrostatic interactions causing the two molecules to assemble.
Extrinsic Pathway Targets
CD95, also known as FAS, is primarily expressed in activated T lymphocytes and natural killer (NK) cells. It can induce apoptosis upon the binding of its ligand CD178 (FasL). This apoptotic influence plays a role in the resolution of immune responses and maintenance of immune homeostasis. CD95 also plays a role in the elimination of virally infected and cancer cells by cytotoxic lymphocytes and NK cells. Table 1 below lists the antibodies against CD95.
Table 1. CD95 Antibodies.
Specificity |
Clone |
Applications |
Target Species |
Publications |
Formats |
Catalog Number |
|---|---|---|---|---|---|---|
|
CD95 |
LOB 3/17 |
FC, IP |
Human |
12 |
Pur., FITC, PE, A647 |
|
|
CD95 |
C02-6C8 |
E |
Human |
0 |
Bio. |
|
|
CD95 |
E06-1A9 |
E |
Human |
0 |
Pur. |
|
|
CD178 |
10F2 |
E, FC, FN, IP |
Human |
7 |
Pur., LE |
|
|
CD178 |
14C2 |
FC, E, IP |
Human |
9 |
Pur., PE, A488, A647 |
|
|
CD178 |
MLF4 |
FC, FN |
Mouse |
3 |
Pur., PE, A488, A647, LE |
Abbreviations: Axxx, Alexa Fluor; LE, low entotoxin; PE, phycoerythrin; FC, Flow Cytometry; E, ELISA; IP, immunoprecipitation; FN, functional assay; IHC-P, immunohistochemistry paraffin; WB, western blotting, Pur., purified.
CD120a (TNFR1) is a ubiquitously expressed transmembrane glycoprotein and member of the tumor necrosis factor receptor superfamily (TNFRSF), which acts as a receptor for TNF-alpha and lymphotoxin-alpha (TNF-beta). Table 2 below lists the antibodies against CD120a. Mouse Anti- Human CD120a Antibody (clone H398) inhibits the biological activity of both natural and recombinant human TNF-alpha and TNF-beta (Thoma et al. 1990 and Dri et al. 1999). The ability to selectively inhibit CD120a and the effect of its activation provide a tool for dissecting apoptotic signaling pathways.
Table 2. CD120a Antibodies.
Specificity |
Clone |
Applications |
Target Species |
Publications |
Formats |
Catalog Number |
|---|---|---|---|---|---|---|
|
CD120a |
H398 |
FC, E, FN |
Human, rabbit |
13 |
Pur., FITC, RPE, A647 |
Abbreviations: Axxx, Alexa Fluor; RPE, R-phycoerythrin; FC, Flow Cytometry; E, ELISA; FN, functional assay; Pur., purified.
CD120b (TNFR2) is expressed on lymphocytes, monocytes and granulocytes. CD120b lacks a cytoplasmic death domain and activates inhibitors of apoptosis (cIAP1 and cIAP2). Binding of TNF-alpha to CD120b has been reported to induce cell death in T lymphocytes while promoting cell survival in renal tubular cells. Table 3 lists the antibodies available targeting CD120b.
Table 3. CD120b Antibodies.
Specificity |
Clone |
Applications |
Target Species |
Formats |
Catalog Number |
|---|---|---|---|---|---|
|
CD120b |
22221 (2/220) |
IHC-F, E, FN, WB |
Human |
Pur. |
|
|
CD120b |
Polyclonal |
E, FN, IF/ICC, WB |
Human |
Pur. |
|
|
CD120b |
Polyclonal |
E, WB |
Human |
Preservative Free |
Abbreviations: FN, functional assay; FC, Flow Cytometry; E, ELISA; IF/ICC, immunofluorescence/immunocytochemistry; IHC-F, immunohistochemistry frozen; WB, western blotting; Pur., purified.
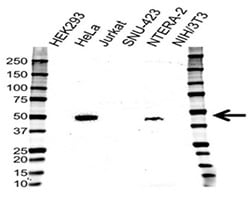
Fig. 2. Western blotting analysis of cell lysates. Probed with CD120b Antibody followed by detection with HRP conjugated Goat Anti-Rabbit (1/10,000, STAR208P). Visualized on the ChemiDoc™ MP with 18 s exposure. Arrow points to CD120b (molecular weight 48 kDa).
Figure 2 shows expression profile of CD120b in a number of cell lines using an antibody highly validated for use in western blotting (VPA00343). Choosing a cell line with robust expression is important when studying the effect of CD120b on apoptosis.
DR3, also known as TNFRSF25, is a cell surface receptor expressed on activated T cells. Activation of DR3 mediates apoptotic signaling and differentiation. The TNFSF ligand for DR3 is TNF-like protein 1A (TL1A). Table 4 shows a list of available DR3 Antibodies.
Table 4. DR3 Antibodies.
Specificity |
Clone |
Applications |
Target Species |
Formats |
Catalog Number |
|---|---|---|---|---|---|
|
DR3 |
Polyclonal |
WB |
Human |
Pur. |
|
|
DR3 |
Polyclonal |
IHC-F, WB |
Human, mouse |
Pur. |
Abbreviations: IHC-F, immunohistochemistry frozen; WB, western blotting; Pur., purified.
CD261, also known as DR4 or TRAIL- R1, is expressed in most human tissues including spleen, peripheral blood leucocytes, thymus and in a variety of tumor-derived cell lines including K-562 and MCF7. The binding of TRAIL to DR4 triggers the activation of pro-caspases-8 and 10, leading to apoptosis. Table 5 lists the antibodies available against CD261.
Table 5. CD261 Antibodies.
Specificity |
Clone |
Applications |
Target Species |
Publications |
Formats |
Catalog Number |
|---|---|---|---|---|---|---|
|
CD261 |
Polyclonal |
WB |
Human |
0 |
Pur. |
|
|
CD261 |
Polyclonal |
IHC-F, WB |
Human |
0 |
Pur. |
Abbreviations: Axxx, Alexa Fluor; IP, immunoprecipitation; FC, Flow Cytometry; IHC-F, immunohistochemistry frozen; WB, western blotting; Pur., purified.
CD262, also known as DR5 and TRAIL-R2, is expressed widely in adult and foetal tissues. Binding of TRAIL to CD262 recruits FADD, induces assembly of the death inducing signaling complex (DISC) and activates the extrinsic apoptotic pathways. Table 6 lists the antibodies against CD262.
Table 6. CD262 Antibodies.
Specificity |
Clone |
Applications |
Target Species |
Formats |
Catalog Number |
|---|---|---|---|---|---|
|
CD262 |
Polyclonal |
IHC-F, WB |
Human |
Pur. |
|
|
CD262 |
DJR2-4 |
FC, IF/ICC |
Human |
Pur. |
Abbreviations: FC, Flow Cytometry; E, ELISA; IF/ICC, immunofluorescence/immunocytochemistry; IHC-F, immunohistochemistry frozen; IHC-P, immunohistochemistry paraffin; Pur., purified; WB, western blotting.
CD266, also known as TNFRSF12A or Fn14, is expressed by a variety of tissues, including heart, lung and kidney and at elevated levels by human brain and liver tumors. CD266 is not expressed on primary T or B cells. Binding of TWEAK to CD266 can stimulate several cellular responses including proliferation, inflammation or apoptosis depending on the cellular context. Table 7 shows a list of the available CD266 antibodies.
Table 7. CD266 Antibodies.
Specificity |
Clone |
Applications |
Target Species |
Publications |
Formats |
Catalog Number |
|---|---|---|---|---|---|---|
|
CD266 |
ITEM-4 |
IHC-F, FC, WB |
Human |
4 |
Pur., FITC, RPE, A488, A647 |
Abbreviations: Axxx, Alexa Fluor; FC, Flow Cytometry; IHC-F, immunohistochemistry frozen; RPE, R-phycoerythrin; Pur., purified; WB, western blotting.
Granzyme Mediated Induction of Apoptosis
Apoptosis of virally-infected cells and primary tumor cells may be induced by cytotoxic T lymphocytes (CTLs) through the release of perforin and granzymes from cytoplasmic cytolytic granules. Granzymes (highly homologous serine proteases) are activated through the cleavage of an amino-terminal dipeptide. Table 8 lists antibodies against granzymes.
Table 8. Antibodies against granzymes.
Specificity |
Clone |
Applications |
Target Species |
Format |
Catalog Number |
|---|---|---|---|---|---|
|
Granzyme A |
GA6 |
IHC-P, WB |
Human |
Pur. |
|
|
Granzyme B |
GB10 |
E |
Human |
B. |
|
|
Granzyme B |
GB11 |
IHC-P, E, FC, IP |
Human |
Pur. |
|
|
Granzyme B |
GB7 |
IHC-P, WB |
Human |
Pur. |
Abbreviations: B., biotin; FC, Flow Cytometry; E, ELISA; IHC-F, immunohistochemistry frozen; IHC-P, immunohistochemistry paraffin; IP, immunoprecipitation; RPE, R-phycoerythrin; Pur., purified; WB, western blotting.
Granzyme A induces caspase-independent cell death characterized by DNA fragmentation. An important target of granzyme A is the endoplasmic reticulum-associated SET complex. The SET complex translocates into the nucleus where the activated DNase NM23-H1 causes characteristic single-stranded DNA damage.
Granzyme B plays a pivotal role in the rapid induction of caspase-dependent apoptosis. In particular, it directly cleaves, and activates, several effector and initiator pro-caspases. Granzyme B also induces caspase-independent events leading to DNA fragmentation in the target cell and mitochondrial dysfunction involving pro-apoptotic cytochrome c release.
Phases and Resources
References
- Dri, P. et al. (1999) Role of the 75-kDa TNF receptor in TNF-induced activation of neutrophil respiratory burst. J Immunol. 162 460-6.
- Thoma, B. et al. (1990) Identification of a 60-kD tumor necrosis factor (TNF) receptor as the major signal transducing component in TNF responses. J Exp Med. 172 1019-23.



