Epithelial to Mesenchymal Transition
EMT Explained
Epithelial to mesenchymal transition (EMT) is a process by which epithelial cells lose their orientation, cell to cell contacts and adopt the mobile characteristics of mesenchymal cells. EMT is not a change between two solid states but rather a process by which cells can move along a phenotypic spectrum (Figure 1). This is demonstrated by the existence of the reverse of EMT, mesenchymal to epithelial transition (MET). Figure 1 shows epithelial cells aligned with other epithelial cells and exhibiting the usual polarity. At this point the EMT inducing stimulus occurs, activating various signaling pathways leading to loss of epithelial gene expression. Following this, cell to cell contacts are removed, cells become more mobile and protease expression increases leading to cells escaping their local environment. Once the cells have reached their new location, the reverse process can occur beginning with an inhibition of mesenchymal gene expression and ending with the return of the cell to an epithelial structure.
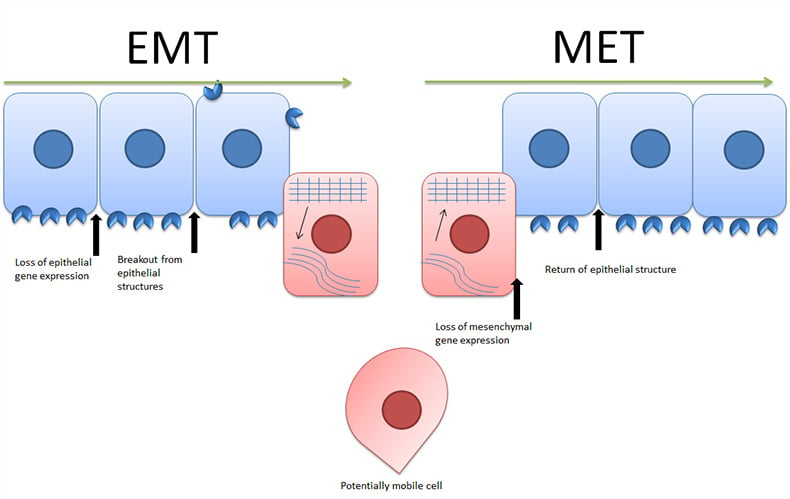
Fig. 1. Epithelial to mesenchymal transition and mesenchymal to epithelial transition.
Induction of EMT
Many factors have demonstrated the ability to induce EMT in vitro including transforming growth factor beta (TGF-β), tumor necrosis factor alpha (TNF-α), hypoxia growth factors and the extracellular matrix (ECM) (Figure 2).
Changes in Gene Expression
In response to the stimuli described above and the associated signaling cascades (SMAD, MAPK, PI3K/Akt), a number of transcription factors are activated. These transcription factors include SNAIL family zinc finger transcription factors, (SNAIL1 and SNAIL2), TWIST family basic helix-loop-helix transcription factors (TWIST1 and TWIST2) and the zinc finger E-box binding homeobox proteins (ZEB1 and ZEB2). The transcription factors decrease the expression of endothelial markers such as E-cadherin and ZO-1 while increasing the expression of mesenchymal markers (vimentin and fibronectin).mesenchymal markers (vimentin and fibronectin).
Loss of Epithelial Characteristics
In addition to the changes in gene expression EMT also induces changes in cell polarity, matrix metalloprotease (MMP) expression and cytoskeletal realignment. The combination of these changes empowers the cell with the ability to detach from its neighbours, escape from the local environment and enter the bloodstream. Once free from the constraints of tertiary epithelial structures the cell can migrate in response to local environmental cues.
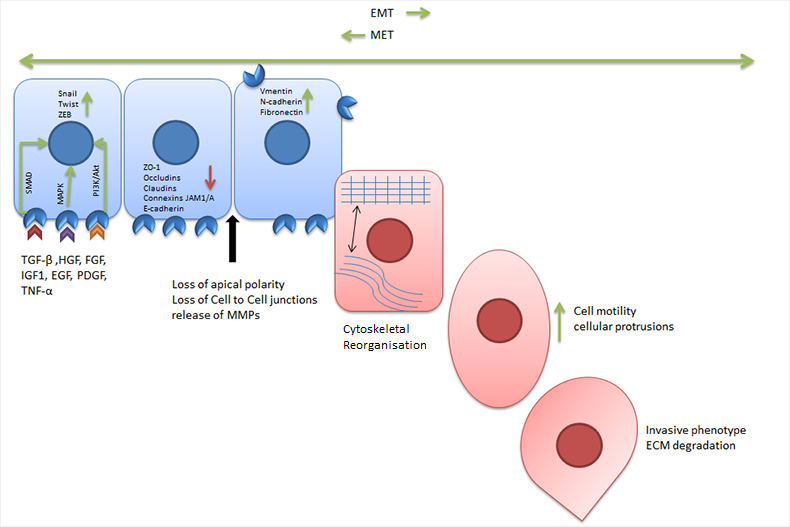
Fig. 2. Overview of epithelial to mesenchymal transition.
EMT in Cancer
EMT has been proposed as a mechanism by which particular epithelial cancers spread from an initial tumor site to secondary sites (metastasis). Under this hypothesis secondary tumors are formed by a cancer cell undergoing EMT, escaping its local environment and relocating. Upon this relocation the reverse process MET would occur. The process of regaining its epithelial characteristics is crucial to the cell being able to infiltrate and anchor within its new tumor site. In addition to producing this metastatic influence, EMT related transcription factors also upregulate anti-apoptotic genes (Grille et al. 2003). This conveys a potential increased resistance to apoptosis, a characteristic that has been defined as a “Hallmark of Cancer”. In clinical environments markers of EMT are being used as potential indicators of treatment resistance and overall patient prognosis (Aktas et al. 2009).
Cellular Models of EMT
While difficult to study in vivo, in vitro approaches to studying EMT and its phenotypical changesmake it relevant to cancer research. Several cell lines if appropriately treated can be induced to undergo EMT. TGF-β treatment is the most common inducer of EMT. Treatment with TGF-β activates EMT in A549 (Kasai et al. 2005), MCF10A (Zhang et al. 2014) and NMuMG (Shirakihara et al. 2007) cell lines.
EMT Transcription Factors
Three transcription factors, TWIST, SNAIL and ZEB are the key effectors of EMT. Members of the TWIST family are basic helix-loop-helix (bHLH) transcription factors. TWIST1 contains several identified phosphorylation sites, the best described is the serine residue 68. This phosphorylation by p38 MAPK prevents the ubiquitin mediated degradation of TWIST1 thereby enabling the transcriptional effects of TWIST1 (Yin et al. 2016). Detection of intact TWIST1 protein is likely to be indicative of TWIST1 activity and ongoing EMT.
Members of the SNAIL family are zinc finger transcription factors capable of inhibiting the transcription of E-cadherin. SNAIL transcription factor expression is regulated by several pathways including TGF-β (Peinado et al. 2007) and by the SMAD family of transcription factors (Sakai et al. 2005). SNAIL1 contains many phosphorylation sites which regulate its activity and stability. Glycogen synthase kinase -3 beta (GSK3B) phosphorylates SNAIL1 at serine residues 97 and 101 leading to export from the nucleus where it is further phosphorylated and degraded (Zhou et al. 2004). Therefore inhibition of GSK3B may be a key step within EMT.
ZEB1 and ZEB2 can repress or activate transcription through binding of regulatory gene sequences at E-boxes. Through this activity ZEB transcription factors are able to contribute to repression of epithelial genes and transcription of mesenchymal genes. The expression of ZEB1/ZEB2 often follows SNAIL/TWIST (Dave et al. 2011) expression and is induced via TGF-BETA and WNT signaling pathways (Xu et al. 2009).
Table 1. Antibodies against EMT related transcription factors.
EMT Transcription Factors |
|||
|---|---|---|---|
|
Target |
Clone |
Applications |
Catalog # |
|
SNAIL |
Polyclonal |
WB |
|
|
Slug (SNAIL2) |
Polyclonal |
WB |
|
|
ZEB1 |
OTI8E2 |
WB |
|
|
SMAD2 |
3G9 |
IF, IHC-P, WB |
|
|
SMAD3 |
Polyclonal |
WB |
|
|
SMAD4 |
1B6 |
WB |
|
|
SMAD4 |
RM277 |
IHC-P, WB |
|
Abbreviations: WB, western blotting; IF, immunofluorescence; IHC-P,immunohistochemistry paraffin.
Detecting Changes in Cellular Phenotype
Several methods including immunofluorescent cell staining and western blotting can be used to verify that EMT has occurred within the cell model. If using immunofluorescence, cells can be probed with antibodies against epithelial markers such as E-cadherin or mesenchymal markers such as vimentin. Cells should have a reduced expression of epithelial and an increased expression of mesenchymal markers. Cells can also be stained for actin and after having undergone EMT will show a more elongated morphology with actin stress fibres and cellular protrusions. Viewing unstained cells should show the loss of the uniform “cobblestone” appearance of epithelial cells and the change to the isolated elongated phenotype of mesenchymal cells.
For western blot analysis of EMT, lysates should be probed with antibodies against epithelial markers such as E-cadherin (Figure 3) or mesenchymal markers such as vimentin. Table 2 lists antibodies that detect the epithelial and mesenchymal markers whose expression changes during EMT. Table 3 list antibodies against cell signaling molecules with a role in EMT.mesenchymal markers such as vimentin. Table 2 lists antibodies that detect the epithelial and mesenchymal markers whose expression changes during EMT. Table 3 list antibodies against cell signaling molecules with a role in EMT.
Table 2. Antibodies against EMT phenotype markers.
Epithelial Markers |
|||||
|---|---|---|---|---|---|
|
Target |
Clone |
Applications |
Catalog # |
||
|
CD324/ E-cadherin |
67A4 |
WB |
|||
|
CD324/ E-cadherin |
AB04/4G6 |
WB |
|||
|
OCLN/occludin |
1G7 |
IF, WB |
|||
Mesenchymal Markers |
|||||
|---|---|---|---|---|---|
|
Target |
Clone |
Applications |
Catalog # |
||
|
Vimentin |
AbD2701 |
IHC-F, IHC-P, IP, E, WB |
|||
|
N-cadherin |
13A9 |
IHC-F, IHC-P, IF, WB, IP |
|||
|
Fibronectin |
875A51 |
WB |
|||
| Fibronectin | Polyclonal | WB | VPA000045 | ||
Abbreviations: E, ELISA; WB, western blotting; FC, flow cytometry; IF, immunofluorescence; IHC-F, immunohistochemistry frozen; IHC-P, immunohistochemistry paraffin.
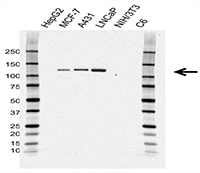
Fig. 3. Western blot analysis of whole cell lysates probed with CD324 (E-Cadherin) Antibody. Primary antibody incubation was followed by detection with HRP conjugated Goat Anti-Mouse IgG (1/10,000, STAR207P) and visualized on the ChemiDoc™ MP Imaging System with 6-second exposure. Arrow points to CD324 (E-Cadherin) (molecular weight 120 kDa).
Table 3. Antibodies against EMT related signaling molecules.
EMT related Signaling Molecules |
|
|||
|---|---|---|---|---|
|
Target |
Clone |
Applications |
Catalog # |
|
|
GSK3B |
PrecisionAb™ |
WB |
||
|
GSK3B |
Polyclonal |
WB |
||
|
FAK |
OTI4A8 |
WB |
||
|
GSK3B pSer9 |
Polyclonal |
WB |
||
|
GSK3B pSer9 |
Polyclonal |
WB |
||
|
p53 |
DO-1 |
WB, IHC-F, IHC-P, IP, E, FC |
||
|
p53 |
DO-7 |
WB, IHC-F, IHC-P, IP, FC |
||
|
p53 |
DO-11 |
WB, IHC-F, IHC-P, IP |
||
|
p53 pSer6 |
Polyclonal |
WB |
||
|
p53 pSer9 |
Polyclonal |
WB |
||
|
p53 pSer392 |
Polyclonal |
WB |
||
|
Catenin Beta |
Polyclonal |
WB |
||
|
Catenin Beta pTyr333 |
Polyclonal |
WB |
||
|
Catenin Beta |
Polyclonal |
WB |
||
|
Catenin Beta |
PrecisionAb |
WB |
||
|
MMP-9 |
4A3 |
IHC-P, WB |
||
|
EZH2 |
PrecisionAb |
WB |
||
|
BMP-2 |
Polyclonal |
E,IF, IHC-P, WB |
||
|
BMP-7 |
Polyclonal |
WB |
||
Abbreviations: WB, western blotting; E, ELISA; FC, flow cytometry; IF, immunofluorescence; IHC-F, immunohistochemistry frozen; IHC-P, immunohistochemistry paraffin; IP, immunoprecipitation.
References
- Aktas et al. (2009). Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumour cells of metastatic breast cancer patients. Breast Cancer Res. 11, R46.
- Dave N et al. (2011). Functional cooperation between Snail1 and twist in the regulation of ZEB1 expression during epithelial to mesenchymal transition. mesenchymal transition. J Biol Chem 14, 12024-32.
- Kasai H et al. (2005). TGF-β1 induces human alveolar epithelial to mesenchymal cell transition (EMT). mesenchymal cell transition (EMT). Respiratory Research 56.
- Grille S et al. (2003). The protein kinase Akt induces epithelial to mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Research 63, 2172.
- Peinado H et al. (2007). Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 6, 415-28.
- Sakai D et al. (2005). Regulation of Slug transcription in embryonic ectoderm by beta-catenin-Lef/Tcf and BMP-Smad signaling. Dev Growth Differ 7, 471-82.
- Shirakihara T et al. (2007). Differential regulation of epithelial and mesenchymal markers by δEF1 proteins in epithelial–mesenchymal transition induced by TGF-β. mesenchymal markers by δEF1 proteins in epithelial–mesenchymal transition induced by TGF-β. Mol Biol Cell 9,3533-3544.
- Xu J et al. (2009). TGF-β-induced epithelial to mesenchymal transition. mesenchymal transition. Cell Res. 2, 156-172.
- Yin J et al. (2016). Prp19 facilitates invasion of hepatocellular carcinoma via p38 mitogen-activated protein kinase/twist1 pathway. Oncotarget 16, 21939-51.
- Zhou BP et al. (2004) Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol 10, 931-40.
- Zhang J et al. (2014). TGF-β–induced epithelial-to-mesenchymal transition proceeds through stepwise activation of multiple feedback loops. Sci Signal 345, 91.



