Immune Senescence and Inflammaging Mini-Review
The last 50 years have seen a steady increase in life expectancy across the developed world mainly due to disease prevention, improved hygiene, and nutrition. However with increasing age, a new problem has appeared - the phenomenon of inflammaging and immune senescence. This is now a focus for research as the decline in immune parameters which can lead to disabilities and poor health in an aging population, has significant impact on social welfare. Inflammaging is a hallmark of virtually every major age-related disease and phenotype such as dementia, Parkinson’s disease, atherosclerosis, type 2 diabetes, cancer, and arthritis, and is a pathological characteristic of aging tissues found across multiple species (Xu and Larbi 2017).

Immune Senescence and Inflammaging - Using flow cytometry to measure the effects of aging on the immune system
Inflammaging Definition and Hallmarks
Inflammaging refers to low levels of chronic inflammation that progressively increase with age. It is thought to be due to the age-related accumulation of senescent cells (here senescent meaning old) which do not proliferate. This is theorized, in part, due to resistance to apoptosis induced damage and not T cell exhaustion. Inflammaging is characterized by low level persistent infiltration of immune cells, primarily but not exclusively, cells of the innate immune system and elevated levels of pro-inflammatory cytokines, and chemokines. With age, this low grade inflammation leads to a counter-regulation of anti-inflammatory molecules. Cells of both the innate and adaptive immune compartments can change with age, becoming senescent. It is not clear why this happens, but may be as a result of sustained activation in the absence of specific challenge and persistence of memory, even in the innate immune compartment (Ventura et al. 2017).
Role of Cellular Stress
Apoptosis is one of many changes typical of immune aging like thymic involution, alteration of the T cell repertoire, and the accumulation of effector memory cells. Without normal levels of apoptotic cell death, there is, amongst other things, an increase in the number of senescent cells. This immunosenescence is responsible for many of the changes seen in the immune system. Senescent cells are cells that have entered a state of irreversibly arrested cell proliferation and altered function as a consequence of many of the stresses that are known to increase with age. Such stresses include genomic or epigenomic damage, oxygen metabolites, oncogene activation, inflammasome activation, defective autophagy, defective ubiquitin/proteasome system, metabolic imbalance, and mitochondrial dysfunction. In addition to cellular stresses, one theory suggests changes to the immune system may also be associated with microbial products translocating from the gut into the circulation, stressing the importance of the gut microbiota on the immune system. It is postulated that although immunosenescence increases human longevity, the pro-inflammatory nature can lead to dementia, Parkinson’s disease, atherosclerosis, inflammatory disease, and neoplastic disease increase (Ventura et al. 2017).
Senescence-Associated Secretory Phenotype
One of the hallmarks of immune aging is the acquisition of a senescence-associated secretory phenotype (SASP). This is where there is induction and secretion of inflammatory cytokines like IL-1 and TNF-α, known to promote leukocyte recruitment which results in a persistent low level subclinical inflammation. The origins of this cytokine and chemokine up-regulation are not fully understood. One suggestion is that altered cellular function caused by the stresses that increase with age, results in sustained activation in the absence of specific challenge and persistence of memory (Ventura et al. 2017). In fact, it has recently been shown that age-associated accumulation of adipose tissue may be the cause of elevated inflammatory cytokines in obese individuals. Furthermore, adiponectin, associated with lean individuals, polarizes macrophages into the anti-inflammatory M2 phenotype. It was even demonstrated that leaner elderly subjects taking regular exercise have fewer senescent T cells and lower pro-inflammatory cytokines (Pinti et al. 2016) emphasizing the benefits of a healthy lifestyle leading to longer lifespans.
The immune system as a whole is constantly replenished from hematopoietic cells in the bone marrow, but this ability declines over time and the total amount of hematopoietic tissue decreases including myeloid, erythroid, and B cell progenitors, contributing to immunosenescence.
We will now take a look at how specific immune cell subsets are affected by inflammaging and senescence.
Innate Immunity
Granulocytes
With age, the absolute number of neutrophils does not change, but they do have reduced capability to migrate to chemotactic signals such as IL-8 and fMLP. A reduced ability of chemotaxis could also lead to reduced egress of neutrophils from inflamed tissue leading to higher local inflammation. It has been reported that neutrophils have lower levels of surface CD16, which can lead to lower superoxide generation and reduced capacity to uptake opsonized particles. Recently the ability to form neutrophil NETs has been shown to decrease with aging which may be one of the reasons for increased susceptibility to infections in the elderly (Pinti et al. 2016). Figure 1 below shows how staining with CD4, CD16, and CD13 allows identification of neutrophils and eosinophils in human peripheral blood. Visit our dedicated granulocyte page for more information on markers to identify granulocyte subsets and their function.
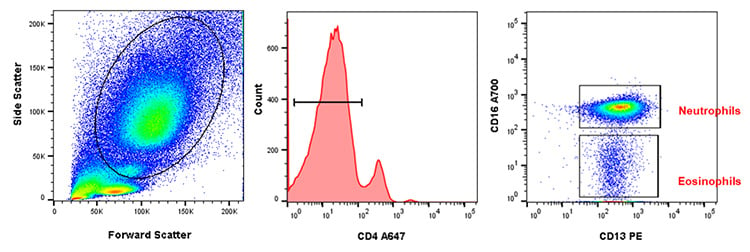
Fig. 1. Neutrophil and eosinophil staining in human peripheral blood. After using forward and side scatter to identify the myeloid population, CD4 (MCA1267P647) was used to distinguish between granulocytes and monocytes. CD16 (MCA2537A700) and CD13 (MCA1270PE) allow identification of CD16+CD13+ neutrophils and CD16-CD13+ eosinophils within the CD4- population.
Monocytes
Monocytes can be divided into three main population subsets (Figure 2): classical (CD14++/CD16-), non-classical (CD14+/CD16++), and intermediate (CD14++/CD16+). Although the exact role of these different monocyte subsets is unclear, non-classical monocytes have been demonstrated to be pro-inflammatory, a hallmark of aging. Monocyte function is probably determined by the external stimuli they receive which may be altered with age. Aging leads to a reduction in the proportion of classical subsets, often with a corresponding increase in the other two. A reduction in creation of reactive oxygen species (ROS) and phagocytosis has been reported, although chemotaxis appears unaffected. Monocytes in the elderly have also been shown to have an altered cytokine secretion pattern after stimulation through pattern recognition receptors (PRRs) such as Toll-like receptors (TLR), NOD-like receptors, and Rig-like receptors (Pinti et al. 2016). Visit our innate immunity page for more information on PRRs and available antibodies.
More in-depth information regarding monocyte markers, lineage, development, and characterization of monocyte subtypes is available via our monocytes, macrophages and dendritic cells page. You may also be interested in the popular macrophage polarization mini-review and a guide to identifying myeloid cells by flow cytometry.
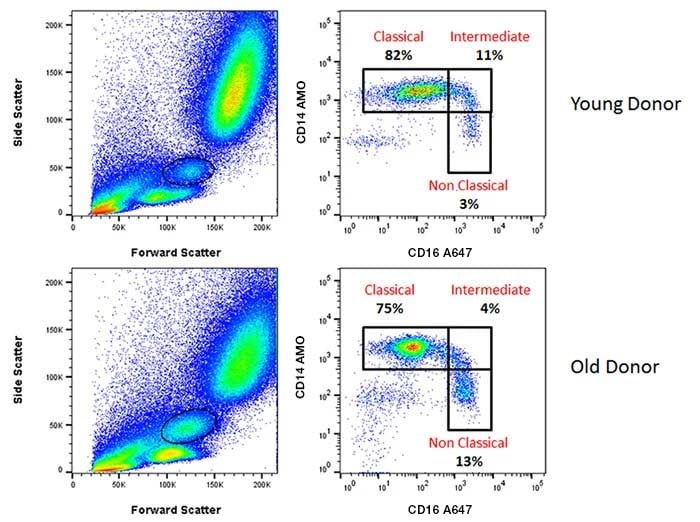
Fig. 2. Monocyte staining in human peripheral blood from young and old donors. CD14 AMO (MCA1568AMO) and CD16 A647 (MCA2537A647) allowed identification of monocyte subpopulations from live, single cell monocytes that were negative for CD19 A488 (MCA1940A488) and CD3 A488 (MCA463A488). Subpopulations identified were CD14++CD16- classical monocytes, CD14++CD16+ intermediate monocytes, and CD14+CD16+ non-classical monocytes.
Dendritic Cells
Dendritic cells (DCs) have been studied in detail but unfortunately show contrasting data due to their diverse development and phenotypes. With increased age, classical myeloid DCs found at sites of microbial encounters, have revealed contrasting cytokine profiles. However, plasmacytoid DCs, located in peripheral organs, display impaired pro-inflammatory cytokine release. Phagocytosis of antigens appears to be unaffected, but DCs seem less able to prime CD4+ T cells. It is not yet known whether this is an effect of or in response to presentation, most likely a combination of both. Langerhans cells, the specialized skin antigen presenting cells, markedly diminish in number with age, which could contribute to increased skin infections seen in aged individuals (Pinti et al. 2016). Visit our dendritic cell webpage for more information on key lineage and identification markers to help you identify both human and murine dendritic cells.
Other cells of the innate immune system such as mast cells, basophils and eosinophils appear to be mostly unaffected by age, or have not yet been studied in detail.
Adaptive Immunity
T cells
Naïve CD4+ helper and CD8+ cytotoxic T cells are generated in the thymus which degenerates with age in a process called thymic involution. In young individuals, naïve cells generally express markers such as CD45RA and CCR7, which allow them to home into lymph nodes, and CD27 and CD28, which are co-stimulatory molecules necessary for interaction with antigen presenting cells. Central memory cells are CD45RO+, capable of producing IL-2 and IFN-γ. Effector memory cells generally produce effector cytokines, like TNF-α, IL-4 and IL-5, while terminal effector cells secrete a wide range of cytokines, but have limited proliferative capacity (Xu and Larbi 2017). The surface expression of common surface markers in CD4+ and CD8+ T cell differentiation in healthy individuals is shown in Table 1 below.
Table 1. Markers of human T cell differentiation.
|
CD4+CD8+ |
CD4+ |
CD4+ |
CD8+ |
CD4+ |
CD8+ |
CD4+CD8+ |
CD4+ |
CD8+ |
|---|---|---|---|---|---|---|---|---|---|
|
Definition |
N |
N |
CM |
CM |
EM |
EM |
EM |
TEMRA |
TEMRA |
|
CCR7 |
|
+ |
+ |
+ |
- |
- |
- |
+ |
+ |
|
CD45RA |
+ |
+ |
- |
- |
- |
- |
- |
+ |
+ |
|
CD28 |
++ |
++ |
++++ |
+++ |
+ |
± |
- |
- |
- |
|
CD27 |
++ |
++ |
+++ |
++++ |
± |
+ |
- |
- |
- |
|
CD57 |
- |
- |
- |
- |
± |
± |
+ |
++ |
++ |
|
CD45RO |
- |
- |
+ |
+ |
+ |
+ |
+ |
± |
± |
|
CD31 |
+ |
- |
- |
- |
- |
- |
- |
- |
- |
|
PD1 |
- |
- |
- |
- |
± |
++ |
++ |
± |
± |
|
KLRG1 |
- |
- |
- |
- |
- |
± |
+ |
++ |
++ |
|
CD127 |
+ |
+ |
++ |
++ |
+ |
+ |
+ |
+ |
+ |
Adapted from Larbi and Fulop, Cytometry Part A, 2014.
Abbreviations: N, naïve; CM, central memory; EM, effector memory; TEMRA, terminal effector.
Effects of aging on T cells
The aging process leads to a general increase in CD8+ numbers, specifically memory cells that have lost CD28 and CD27 expression, but gained KLRG1 and CD57. Aging also leads to a reduction in the frequency of CD45RA naïve T cells, together with an increased proportion of terminally differentiated CD45RO effector and memory T lymphocytes, as well as an increase in CD25+ FOXP3+ Tregs. Figure 3 below shows the proportion of naïve and memory T cells in peripheral blood based upon CD45RA, CD45RO, and CD57 staining. These changes are common in blood, as well as lymphoid tissues and organs of older people, and are thought to be responsible for the increased incidence of infections, cancers, reactivation of chronic infections, and failure of vaccinations. However this is not conclusive, as stem cell memory cells may replenish the naïve compartment and cancer incidence plateaus after 90 years of age. Furthermore there are other factors influencing vaccine efficacy such as the adjuvant used in combination with the antigen. Senescence related reduction in the naïve T cell compartment is associated with a 2-5 fold less diverse TCR repertoire. However, with the decrease of the repertoire, there is an increase of large T cell clones suggesting uneven homeostatic proliferation. There is an increase in terminally differentiated T cells in the elderly which is thought to be the result of recurrent or chronic infections, and immune activation. These terminally differentiated T cells (CD28-/CD57+) have been shown to have stronger effector functions such as cytolytic capacity and an increased pro-inflammatory profile (Fulop et al. 2018).
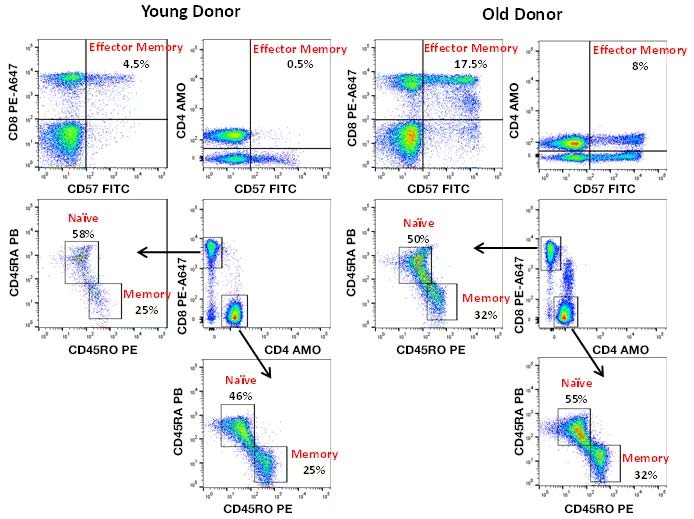
Fig. 3. Naïve and memory T cell identification in human peripheral blood. CD3 A700 (MCA463A700), CD4 AMO (MCA1267AMO), CD8 PE-A647 (MCA1226P647), CD57 FITC (MCA1305F), CD45RA PB (MCA88PB), and CD45RO PE (MCA461PE) staining of human peripheral blood allows identification of naïve, memory and effector memory helper and cytotoxic T cells. Fewer CD45RO+ memory and CD57+ effector memory T cells are observed in young donors compared to old donors.
Checkpoints and Functional Defects
As mentioned earlier, senescent T cells have a reduced proliferative capacity. The intensity of immune responses can be regulated by immune checkpoints like PD-1, CTLA-4, LAG-3, and TIM-3, allowing the immune system to adapt to the levels of antigenic stimulation. This limits the immune alterations associated with aging, but also the effectiveness of the immune response in the setting of malignancy. Visit our dedicated webpage for an in-depth look at immune checkpoints.
So far identified functional defects include altered TCR signaling and TCR-induced ERK phosphorylation, resulting in a reduced capacity to induce de novo antigen specific T cell responses and shortening of telomeres. With the loss of CD27 and CD28, there is an up-regulation of p16 and p21, inhibiting CDK4 and CDK6, which leads to poor transition from G1 to S phase of the cell cycle. Similar to the innate immune system, the acquired immune system has shown defective mitochondrial function, elevated ROS levels, and alterations to energetic metabolism and autophagy which impacts on NF-κB dependent gene expression. NF-κB regulates genes responsible for pro-inflammatory cytokines such as TNF-α, IL-1, and IL-6, thought to be responsible for many of the hallmarks of aging (Bektas et al. 2017).
Non-classical T Cells
In addition to αβ T cells, the γδ T cell compartment is also affected with age. There is a total decline in cell frequency, an accumulation of highly differentiated cells, and changes to the TCR repertoire. How other T cell subsets, like mucosal associated invariant T cells, are affected is still to be elucidated in part due to the inability to use the same markers to identify and classify these cells as with classical CD4+ and CD8+ T cells (Xu and Larbi 2017).
Visit our T cell webpage for more information on helper cell subsets, the T cell receptor, and identification markers to help you identify both human and murine T cells.
B Cells
Although B cells can be antigen presenting cells, they have a primary function to produce antibodies upon stimulation by T cells. Circulating B cell numbers diminish with age resulting in decreased production of high affinity protective antibodies. While this could be due to the lack of sufficient T cell help, T independent responses, such as those to polysaccharides, are also diminished (Ventura et al. 2017).
Figure 4 below shows the four major B cell subsets identifiable by CD19, CD27, and IgD expression. These are naïve (IgD+CD27-), unswitched memory (IgD+CD27+), switched memory (IgD-CD27+), and late memory (IgD-CD27-). Studies have demonstrated that switched memory B cells diminish with age. Conversely the proportion of late memory B cells increases with age. Plasma cell numbers also seem to be reduced in the bone marrow of the elderly. Late memory B cells do not proliferate, but they are transcriptionally active and express the highest level of SASP markers, like TNF-α, IL-6, and IL-8, within memory B cell subsets. The increase in this subset is probably due to terminal differentiation of subsets that have undergone class switching after chronic exposure to antigens (Frasca 2017). In addition to B cells, the levels of serum IgM and IgD have been reported to be reduced with an increase in IgG isotypes. Concurrent with a change in surface markers, specific functions of B cells in the elderly change. Activation-induced cytidine deaminase (AID) and class-switch recombination transcripts are reduced in the aged immune system leading to reduced ability to produce an influenza vaccine specific response. This is probably through impaired affinity maturation which may be caused by elevated TNF-α levels (Blomberg and Frasca 2013).
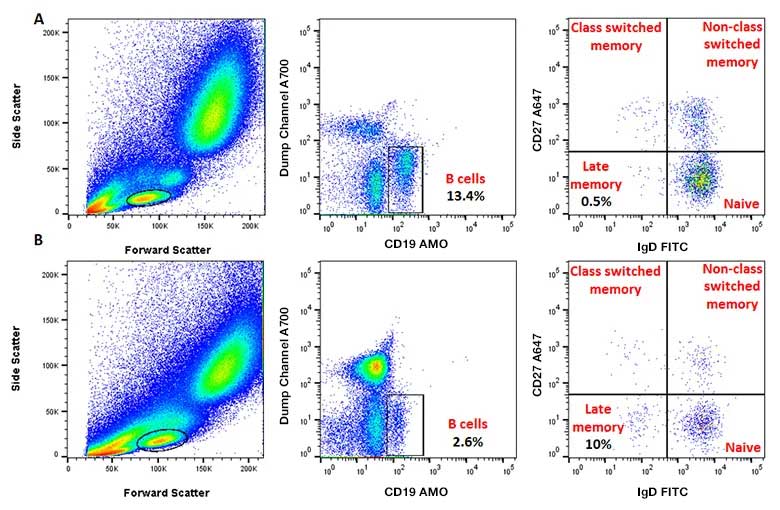
Fig. 4. B cell staining in human peripheral blood. CD27 A647 (MCA755A647) and IgD FITC (STAR143F) allow identification of naïve, class switched and non-class switched memory and late memory (also called exhausted) B cells within the CD19 AMO (MCA2495AMO) positive, CD3 A700 (MCA463A700), and CD14 A700 (MCA1568A700) negative population. A, staining of a young donor with more total B cells and fewer late memory cells compared to an old donor in B.
Visit our B cell webpage for more information on markers to identify B cells, a mini-review and a flow cytometry guide to B cell immunophenotyping.
Natural Killer Cells
Investigations into how natural killer (NK) cells change with age have shown inconsistent findings. The proportion of CD56++ NK cells appears to be reduced with age, but this is probably due to limited production of precursors as mentioned earlier. So far studies have shown inconsistent numbers and percentages of CD56+CD16+, and CD56- NK cells with aging, with either elevated or diminished counts, depending upon the study. However a breakdown in NK cell diversity could influence immune surveillance and therefore be relevant in the context of cancer development and viral infections which are more prominent in the elderly (Pinti et al. 2016). Visit the dedicated NK cell page for a NK cell mini review, information on activating and inhibitory receptors, NK cell development, and NK cell markers.
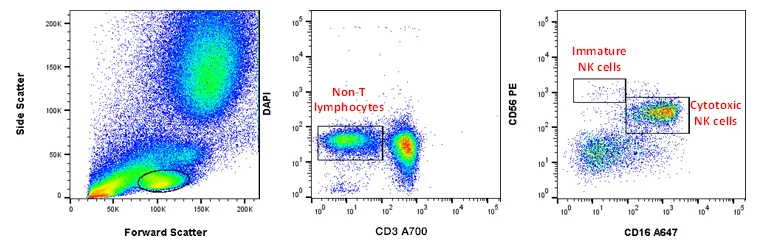
Fig. 5. NK cell staining in peripheral blood. CD56 A488 (MCA2693A488) and CD16 A647 (MCA1193A647) staining of human peripheral blood allow identification of circulating NK cells, which can be split into CD56dim CD16+ mature cytotoxic NK cells, and CD56bright CD16neg/dim immature NK cells which have a cytokine-mediated immune-modulatory role.
Conclusion
In conclusion, there are many changes that occur with inflammaging and senescence, but the reason for these changes has yet to be fully elucidated. In addition to the cellular changes described, there are many other alterations to signaling and metabolism, including responses to glucose levels, but is beyond the scope here. More information can be found in the references at the end of this article.
Furthermore, is inflammaging the cause of the changes or the response to it? The modifications of the immune system may be adaptations to cope with pathogens in a local environment to conserve the resources of an aging body and have therefore been an evolutionary adaptation to avoid excessive energy requirements. Maintaining the balance between inflammaging and an ability to respond to pathogens and the checkpoints that regulate the immune response may in fact be more important for healthy old age than rejuvenation. As our understanding of this highly complex process increases, more effective treatments of age-related diseases are likely to become available.
References
- Bektas A et al. (2017). Human T cell immunosenescence and inflammation in aging. J Leukocyte Biol 102(4), 977-988.
- Blomberg BB and Frasca D (2013). Age effects on mouse and human B cells. Immunol Res 57(1-3), 354-360.
- Frasca D (2017). Senescent B cells in aging and age-related diseases: their role in the regulation of antibody responses. Experimental Gerontology 107, 55-58.
- Fulop T et al. (2018). Immunosenescence and Inflamm-aging as two sides of the same coin: Friends or Foes? Frontiers in Immunology 8, 1960.
- Larbi A and Fulop T (2014). From truly naïve to exhausted senescent T cells: when markers predict functionality. Cytometry Part A 85(1), 25-35.
- Pinti M et al. (2016). Aging of the immune system – focus on inflammation and vaccination. Eur J Immunol 46(10), 2286-2301.
- Ventura MT et al. (2017). Immunosenescence in aging: between immune cells depletion and cytokine up-regulation. Clinical and Molecular Allergy 15, 21.
- Xu W and Larbi A (2017). Markers of T cell senescence in humans. International Journal of Molecular Sciences 18(8), 1742.








