Intracellular Flow Cytometry

- On This Page
- Fixation
- Permeabilization
- Intracellular Staining Tips
- Antibodies
Optimization of Intracellular Flow Cytometry Staining Results with Leucoperm
Flow cytometry protocols and staining procedures vary depending on whether the antigen to be detected is located on the cell surface or intracellular. Detection of cell surface proteins, such as CD markers, is relatively straightforward and apart from blocking of Fc receptors the protocol requires no extra steps. However, when staining intracellular proteins such as cytokines or transcription factors, both fixation and permeabilization steps are required prior to antibody staining.
Fixation
A fixation step is essential to preserve the cellular morphology. Without it the cells will lyse when permeabilized. The choice of fixative is an important first step. Formaldehyde, which creates bonds between lysine residues to cross-link proteins, is commonly used. It is used at concentrations ranging from 0.5%-4% depending on the target antigen and cell type. However, higher concentrations can lead to increased autofluorescence. Alcohols at concentrations of 70% can also be used for fixation. They work by precipitating proteins and allow long term storage at 4oC or -20oC. They have the added benefit of permeabilizing lipid membranes but the denaturing process can lead to epitope masking therefore some optimization of your fixation process may be needed.
Permeabilization
In order for the antibody to penetrate the plasma membrane the membrane must first be permeabilized. This is usually performed using detergents, with the epitope location determining the choice of detergent to be used. Stronger detergents are required to allow staining of nuclear epitopes as both the plasma membrane and nuclear membranes have to be permeabilized. Often for nuclear staining, alcohols are used as they are stronger permeabilization reagents. We also recommend including permeabilization buffer in your washes to remove excess antibody and prevent high background levels.
For staining of intracellular antigens, Bio-Rad offers Leucoperm buffer set (BUF09), which contains fixative and permeabilization reagents that have been optimized to guarantee maximal staining without altering the cellular morphology. A list of flow cytometry tested antibodies for which we highly recommend the use of the Leucoperm buffer set, BUF09, can be found below.
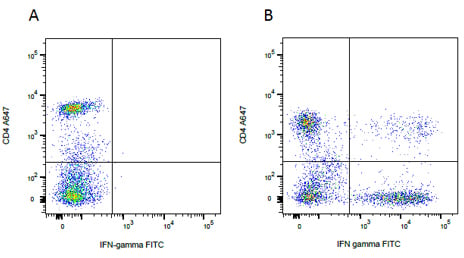
Intracellular staining. Human whole blood was surface stained for CD4A647 (MCA1267A647) followed by intracellular staining for IFN- gamma FITC (MCA1554F) after treatment with Leucoperm (BUF09), on (A) resting lymphocytes or (B) lymphocytes stimulated with PMA/Ionomycin and treated with Monensin prior to staining.
Intracellular Staining Tips
In addition to carefully choosing the most suitable fixative and permeabilzation reagents, the following factors should be considered when designing intracellular flow experiments:
- Not every intracellular antigen staining protocol is the same. Different staining procedures should be used depending on whether the protein to be detected is a cytokine, a transcription factor or a phosphorylated protein
- Surface markers should be stained first then the cells fixed and permeabilized to avoid any potential effects of the intracellular staining protocol
- For staining of secreted proteins, such as cytokines, a protein transport inhibitor, such as Monensin or Brefeldin A, should be added prior to fixation/permeabilization in order to trap the cytokines inside the cells and enable intracellular staining. Different inhibitors are recommended for different types of cytokines and for the detection in different species (e.g. human versus murine cells)
- The fluorophore chosen can be affected by the staining protocol. The size of the fluorophore and fixative used may alter the expected results. For example, PE is a large protein which may not reach all epitopes and can be affected by alcohol fixation. Some fluorophores have also been shown to bind non-specifically (e.g. FITC)
- When simultaneous detection of surface markers and transcription factors is required in a flow cytometry panel, specific transcription factor buffers should be used. The reason for this being that fixation/permeabilization steps can change/weaken cell surface marker staining. Transcription factor buffer sets ensure the detection of intracellular antigens without having adverse effects on the staining of surface markers
- Phosphorylation is a highly transient process and counteracted by numerous phosphatases. In order to detect phosphorylation after stimulation, cells should be immediately fixed and permeabilized
- The brightness of tandem dyes might be reduced by a fixation or permeabilization step. If tandem dye conjugated antibodies are part of your intracellular flow cytometry panel, fixation and permeabilization steps should be as mild as possible and the duration as short as possible
Antibodies for which Leucoperm™ permeabilization is recommended prior to staining
| Description | Target | Format | Clone | Applications | Citations | Code |
|---|






