mTOR Signaling Pathway

- On This Page
- mTOR signaling pathway
- Interactive mTOR signaling pathway poster
- Our mTOR signaling antibody range
- References
A central regulator of cell metabolism, growth, proliferation, and survival
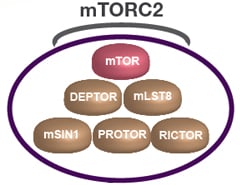
Fig. 1. Schematic drawing of the mTORC2 complex. mTORC2 contains mTOR, DEPTOR, mLST8, RICTOR, mSIN1, and PROTOR (Populo et al. 2012).
The mammalian target of rapamycin (mTOR) is a serine/threonine kinase, which forms the core component of mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) (Bhat et al. 2015) (Figure 1). The complexes differ structurally and functionally, and have different sensitivity to the macrolide rapamycin.
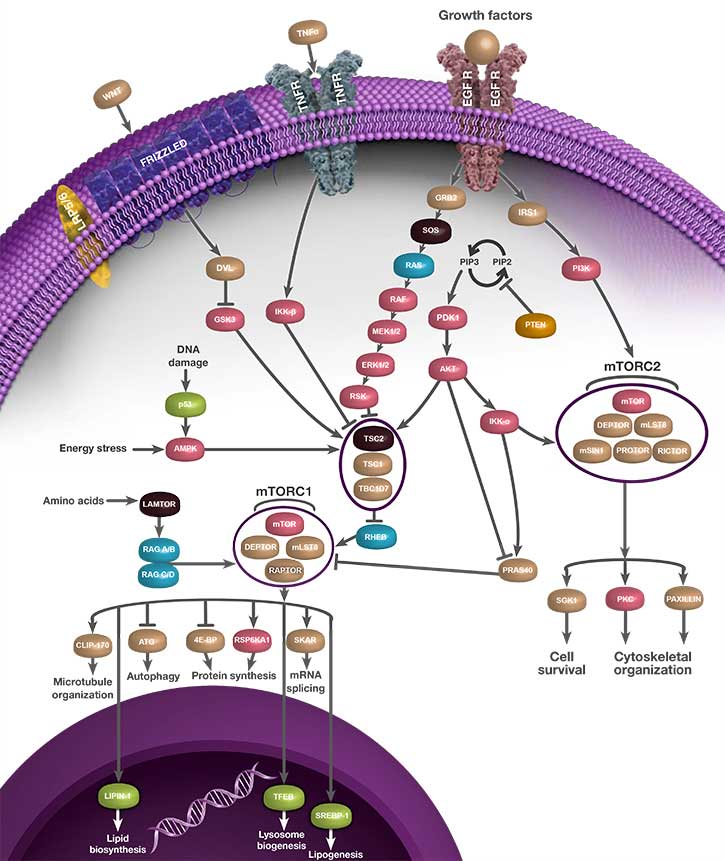
mTORC1 regulates cell growth and proliferation by controlling protein synthesis, lipid synthesis, autophagy, and metabolic programs (Bhat et al. 2015 and Laplante and Sabatini 2012) and can be activated by various stimuli such as growth factors, nutrients, energy, and stress signals (Populo et al. 2012). The mTORC1 complex consists of mTOR, DEP domain-containing mTOR-interacting protein (DEPTOR), mammalian lethal with SEC13 protein 8 (mLST8), proline-rich AKT1 substrate 1 (PRAS40), and regulatory-associated protein of mTOR (RAPTOR) (Figure 2). mTOR is the catalytic subunit of the complex. DEPTOR binding to mTOR negatively regulates its kinase activity (Varusai and Nguyen 2018). mLST8 binding to the mTOR kinase domain stabilizes the RAPTOR-mTOR interaction (Kim et al. 2003). PRAS40 regulates mTORC1 kinase activity as a direct inhibitor of substrate binding (Wang et al. 2007). RAPTOR binding mediates mTOR’s function as a scaffold in the mTOR-signaling cascade and binds directly to mTOR (Hara et al. 2002).
One key role for mTOR is that of a negative regulator of autophagy (Mizushima et al. 2008). mTORC1 regulates this process by phosphorylation and suppression of the ULK-Atg13-FIP200 kinase complex, which is an essential mediator of autophagy. Inhibition of mTORC1 leads to autophagosome formation, which subsequently fuses with lysosomes (Jung et al. 2009; Laplante and Sabatini 2012). The death-associated protein 1 (DAP1) is also an mTOR substrate that negatively regulates autophagy (Koren et al. 2010).
Inhibition of mTORC1 kinase activity causes reduction in DAP1 phosphorylation, and converts the protein into an active suppressor of autophagy (Koren et al. 2010).
Information about the importance of the complex in the regulation of protein synthesis can be found on our translation factors page.
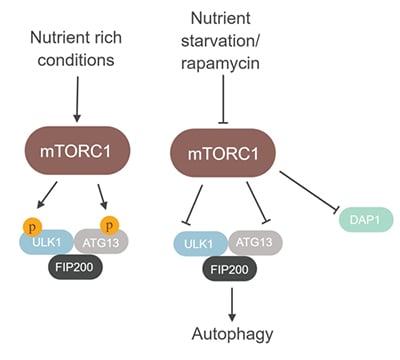
Fig. 2. The role of the mTORC1 complex in the regulation of autophagy. mTORC1 directly phosphorylates and inhibits the ULK-Atg13-FIP200 complex when nutrients are abundant. Phosphorylation of ULK1 and ATG13 prevents induction of autophagy. Upon starvation or rapamycin treatment, mTORC1 is inhibited, which enables the induction of autophagy (Jung et al 2009; Laplante and Sabatini 2012).
The mTORC2 complex regulates cell survival and cytoskeletal organization (Oh and Jacinto 2011) and can be activated by growth factor binding. The complex consists of mTOR, Deptor, mLST8, rapamycin-insensitive companion of mTOR (RICTOR), stress-activated protein kinase-interacting protein 1 (mSIN1), and protein observed with RICTOR (PROTOR) (Populo et al. 2012) (Figure 1). DEPTOR negatively regulates mTORC2 complex (Peterson et al. 2009). Knockout of mLST8 in mice reduces the activity of the complex (Guertin et al. 2006). RICTOR is a key component of the mTORC2 complex and interacts with PROTOR (Woo et al. 2007). It is overexpressed in various cancer types associated with poor survival outcomes (El Shamieh et al. 2018). The RICTOR and mTOR kinases both phosphorylate AKT at serine 473 (Hresko and Mueckler 2005; Sarbassov et al. 2005) as well as facilitate PDK1 mediated phosphorylation at AKT threonine residue 308 (Sarbassov et al. 2005). In addition, mSin1 has also been reported to play a role in mTORC2's capacity to phosphorylate AKT (Frias et al. 2006). PROTOR is a RICTOR-binding subunit of mTORC2 (Pearce at al. 2007) and is required for SGK1 activation in the kidney (Pearce et al. 2011).
Mutations in the mTOR signaling pathway as well as aberrant mTOR signaling have been associated with the development of many diseases including cancer (Populo et al. 2012). Therefore, mTOR represents an attractive therapeutic target (Bhat et al. 2015). Many mTOR inhibitors are licenced FDA-approved drugs and many more are currently being tested in clinical trials.
Interactive mTOR Signaling Pathway Poster
We have compiled key antibodies involved in the mTOR signaling pathway into one handy poster you can download for your reference.
Click the pathway components within the images below to discover the key antibodies that Bio-Rad provides.
Our mTOR signaling antibody range
Browse our range of antibodies against targets involved with mTOR signaling below. Please use the filters to sort the attributes in the table below in order to find the antibody that fits your exact requirements. If you need any further assistance please do not hesitate to contact us.
Antibodies against targets involved with mTOR Signaling
| Description | Target | Format | Clone | Applications | Citations | Code |
|---|
- Growth factors
- EGF R resources
- Kinases
- Transcription factors
- Translation factors
- Phosphatases
- Phosphatases in cancer
- Phospho-Specific precisionAb antibodies
- Phospho-specific antibodies
- Sumoylation mediated signaling
- Ubiquitination mediated signaling
- Tyrosine phosphorylation of EGF R
- Signal transduction antibodies
References
- Bhat M et al. (2015). Targeting the translation machinery in cancer. Nat Rev Drug Discov 14, 261-78.
- El Shamieh S (2018). RICTOR gene amplification is correlated with metastasis and therapeutic resistance in triple-negative breast cancer. Pharmacogenomics 19, 757-760.
- Guertin DA et al. (2006). Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell.11(6), 859-71.
- Frias MA et al. (2006). mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol 16, 1865-70.
- Hara K et al. (2002). Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110, 177-89.
- Hresko RC and Mueckler M (2005). mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem 280, 40406-16.
- Jung CH et al. (2009). ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell 20, 1992-2003.
- Kim DH et al. (2003). GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell 11, 895-904.
- Koren I et al. (2010). DAP1, a novel substrate of mTOR, negatively regulates autophagy. Curr Biol 20, 1093-8.
- Laplante M and Sabatini DM (2012). mTOR signaling in growth control and disease. Cell 149, 274-93.
- Mizushima N et al. (2008). Autophagy fights disease through cellular self-digestion. Nature 451, 1069-75.
- Oh WJ and Jacinto E (2011). mTOR complex 2 signaling and functions. Cell Cycle 10, 2305-16.
- Pearce LR et al. (2007). Identification of Protor as a novel Rictor-binding component of mTOR complex-2. Biochem J 405, 513-22.
- Pearce LR et al. (2011). Protor-1 is required for efficient mTORC2-mediated activation of SGK1 in the kidney. Biochem J 436, 169-79.
- Peterson TR et al. (2009). DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 137(5), 873-86.
- Populo H et al. (2012). The mTOR signalling pathway in human cancer. Int J Mol Sci 13, 1886-918.
- Sarbassov DD et al. (2005). Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307(5712), 1098-101.
- Varusai TM and Nguyen LK (2018). Dynamic modelling of the mTOR signalling network reveals complex emergent behaviours conferred by DEPTOR. Sci Rep 12, 8(1), 643.
- Wang L et al. (2007). PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J Biol Chem 282, 20036-44.
- Woo SY et al. (2007). PRR5, a novel component of mTOR complex 2, regulates platelet-derived growth factor receptor beta expression and signaling. J Biol Chem. 282(35), 25604-12.


