A Hard AKT to Follow — High Quality Antibodies for AKT Cell Signaling

- On This Page
- AKT cell signaling pathway
- Interactive PI3K/AKT Signaling Pathway Poster
- Bio-Rad’s PI3K/AKT signaling antibody range
- References
AKT Cell Signaling Pathway
The phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway controls apoptosis, cell proliferation, and the cell cycle (Rodon et al. 2013). The serine/threonine kinase AKT is a downstream effector of phosphatidylinositol 3-OH kinase (PI3K) (Burgering and Coffer 1995). Extracellular signals activate PI3K to generate phosphatidylinositol 3′ phosphate (PIP3). The tumor suppressor PTEN is a major negative regulator of the pathway by dephoshorylating the 3’phosphate of PIP3 (Sulis and Parsons 2003). AKT has a PH domain at the N-terminus which is required for the binding of PIP3 (Bhaskar and Hay 2007) (Figure 1). In mammals, there are three AKT isoforms (AKT1, AKT2, and AKT3). Phosphorylation of Thr308 and Ser473 are required for full activation of AKT1. Thr308 phosphorylation is mediated by phosphoinositide-dependent kinase-1 (PDK1) (Vanhaesebroeck and Alessi 2000), whereas Ser473 is phosphorylated by the mammalian target of rapamycin complex 2 (mTORC2) (Sarbassov et al. 2005, Laplante and Sabatini 2012) (Figure 1). AKT activity is inhibited by dephosphorylation by phosphatases including protein phosphatase type 2A (PP2A) (Millward et al. 1999) and pleckstrin homology domain leucine-rich repeat protein phosphatase (PHLPP) (Brognard et al. 2007).
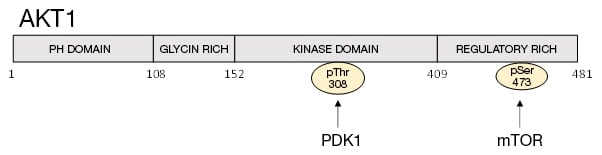
Fig. 1. Structure of AKT1. Position of pThr308 and pSer473 are indicated (Manning and Toker 2017, Kitagishi et al. 2012).
When activated, AKT impacts many cellular processes. It activates the mammalian target of rapamycin complex 1 (mTORC1), which results in increased protein synthesis and cell survival (Aoki et al. 2001). AKT phosphorylates and inhibits Forkhead box O (FOXO) transcription factors, resulting in their degradation in the cytoplasm (Zhang et al. 2011). Phosphorylation of the BCL2 Antagonist of Cell Death (BAD) suppresses apoptosis and promotes cell survival (Datta et al. 1997). Moreover, AKT activates inhibitor of nuclear factor (NF) kappa-B kinase (IKK), activating the NF-kB pathway, and resulting in transcription of antiapoptotic genes (Pommier et al. 2004). AKT also phosphorylates MDM2, allowing its translocation to the nucleus where it targets p53 for degradation (Zhou and Hung 2002). AKT also potently induces Ser-133 phosphorylation of cyclic AMP-response element binding protein (CREB). This phosphorylation promotes the recruitment of co-activator protein CREB binding antibody (CBP) (Du and Montminy 1998) and transcription of genes containing CREs (cAMP-responsive elements) in the promoter.
The PI3K/AKT pathway is also important for the regulation of cell cycle progression. AKT inactivates glycogen synthase kinase 3β (GSK3β), leading to increased cyclin D1 (Liang and Slingerland 2003). It also phosphorylates cell cycle inhibitors p21Waf1/Cip1 and p27Kip1 in order to induce their cytoplasmic retention (Testa and Bellacosa 2001).
Dysfunction of the pathway has been implicated in various pathological conditions including cancer, cardiovascular disease, type 2 diabetes, and autoimmune and neurological disorders (Manning and Toker 2017). Dissecting the complexity of PI3K/AKT signaling offers valuable insights into the development of novel therapeutics for treatment of various disorders.
Interactive PI3K/AKT Signaling Pathway Poster
We have compiled key antibodies involved in the PI3K/AKT signaling pathway into one handy poster you can download for your reference.
Click the pathway components within the images below to discover the key antibodies that Bio-Rad provides.
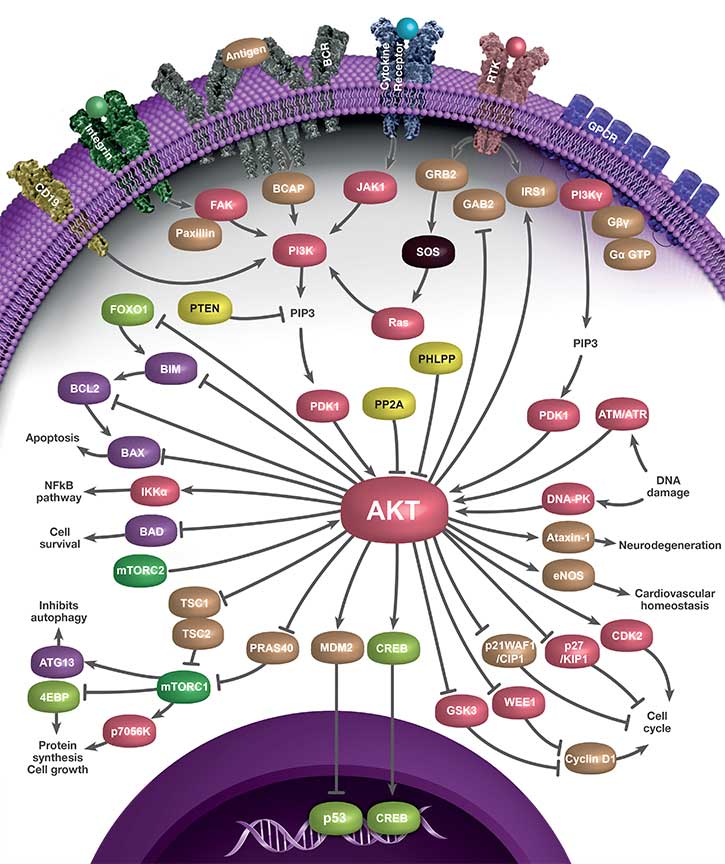
Bio-Rad’s PI3K/AKT Signaling Antibody Range
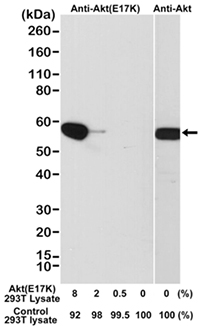
The Rabbit Anti-AKT E17K Mutant Antibody (MCA6297, Figure 2) detects AKT1 kinase mutated at E17K, common in tumorigenesis, where glutamate is substituted for lysine at the 17th amino acid position, in its pleckstrin homology (PH) domain (Carpten et al. 2007). The E17K mutation activates AKT1 by means of pathological localization to the plasma membrane, and stimulates downstream signaling, leading to cellular transformation and tumor formation (Carpten et al. 2007, Brugg et al. 2007). AKT1 E17K mutant triggers phosphorylation and cytoplasmic delocalization of the cyclin-dependent kinase inhibitor p27, and promotes cell proliferation (De Marco et al. 2015). AKT E17K mutant was also found to display higher levels of ubiquitination than AKT wild type. Interestingly, blocking this ubiquitination results in reduction in AKT membrane recruitment and phosphorylation (Yang et al. 2009, Restuccia and Hemmings 2009).
Fig. 2. Western blot analysis of a cell lysate mixture of untransfected 293T and 293T transfected with a DNA construct encoding the AKT E17K mutant and probed with Rabbit Anti-AKT (E17K Mutant) (MCA6297) or Rabbit Anti-AKT (MCA6307).
The mutation is present in multiple cancers, including human breast, ovarian, colorectal (Carpten et al. 2007), lung (Malanga et al. 2008), and endometrial cancer (Cohen et al. 2010). AKT1 E17K may predict clinical response to capivasertib (AZD5363), a drug targeting the AKT signaling pathway (Tamura et al. 2016).
Browse Bio-Rad’s range of antibodies against targets involved in the PI3K/AKT pathway below. Please use the filters to sort the attributes in the table below in order to find the antibody that fits your exact requirements. If you need any further assistance, please do not hesitate to contact us.
High Quality Antibodies for AKT Cell Signaling
| Description | Target | Format | Clone | Applications | Citations | Code |
|---|
References
- Aoki M et al. (2001). A role of the kinase mTOR in cellular transformation induced by the oncoproteins P3k and Akt. Natl Acad Sci USA 98, 136-41.
- Bhaskar PT and Hay N (2007). The two TORCs and Akt. Dev Cell 12(4), 487-502.
- Brognard et al. (2007). PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell 25(6), 917-31.
- Brugg J et al. (2007). A new mutational AKTivation in the PI3K pathway. Cancer Cell 12, 104- 107.
- Burgering BM and Coffer PJ (1995). Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376, 599-602.
- Carpten JD et al. (2007). A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature 448, 439-444.
- Cohen et al. (2010). AKT1 pleckstrin homology domain E17K activating mutation in endometrial carcinoma. Gynecol Oncol 116(1), 88-91.
- Datta SR et al. (1997). Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91(2), 231-41.
- DeMarco C et al. (2015). Mutant AKT1-E17K is oncogenic in lung epithelial cells. Oncotarget 6(37), 39,634-39,650.
- Du K and Montminy M (1998). CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem 273(49), 32,377-32,379.
- Kitagishi Y et al. (2012). Roles of PI3K/AKT/GSK3/mTOR pathway in cell signaling of mental illnesses. Depress Res Treat 752,563.
- Laplante M and Sabatini DM (2012). mTOR signaling in growth control and disease. Cell 149, 274–293.
- Liang J and Slingerland J (2003). Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle 2(4), 339-345.
- Malanga D et al. (2008). Activating E17K mutation in the gene encoding the protein. kinase AKT1 in a subset of squamous cell carcinoma of the lung. Cell Cycle 7(5), 665-669.
- Manning BD and Toker A (2017). AKT/PKB signaling: navigating the network. Cell 169(3), 381-405.
- Millward TA et al. (1999). Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem Sci 24(5), 186-91.
- Pommier et al. (2004). Apoptosis defects and chemotherapy resistance: molecular interaction maps and networks. Oncogene 23, 2,934-2,949.
- Restuccia DF and Hemmings BA. (2009). Blocking Akt-ivity. Science 325(5944), 1,083-1,084.
- Rodon J et al. (2013). Development of PI3K inhibitors: lessons learned from early clinical trials. Nat Rev Clin Oncol 10(3), 143-153.
- Sarbassov DD et al. (2005). Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1,098-1,101.
- Sulis ML and Parsons R. (2003). PTEN: from pathology to biology. Trends Cell Biol 13, 478-483.
- Tamura K et al. (2016). Safety and tolerability of AZD5363 in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol 77(4), 787-95.
- Testa JR and Bellacosa A (2001). AKT plays a central role in tumorigenesis. Proc Natl Acad Sci USA 98(20), 10,983-10,985.
- Vanhaesebroeck B and Alessi DR (2000). The PI3K-PDK1 connection: more than just a road to PKB. Biochem J 346, 561-576.
- Yang WL et al. (2010). Regulation of Akt signaling activation by ubiquitination. Cell Cycle 9:3, 486-497.
- Zhang X et al. (2011). Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta 1813(11), 1978-86.
- Zhou BP and Hung MC (2002). Novel targets of Akt, p21(Cipl/WAF1), and MDM2. Semin Oncol 29, 62-70.