Phospho-Specific Antibodies

- On This Page
- For analyzing cell signaling pathway activation
- Our phospho-specific antibody range
- References
s
Western Blot University
s
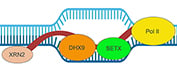
What are R-loops?
s
Cancer Pathway Posters
Compiling key proteins involved in key signaling pathways: cGAS-STING, EGF R, mTOR, NF-kB, and PI3/AKT pathways.
s
Recent Blog
For Analyzing Cell Signaling Pathway Activation
Phosphorylation, the addition of a phosphate group to serine, threonine or tyrosine residues, is an important cellular process utilized to send cellular signals from the membrane to the nucleus. For example, changes in physiological conditions may require immediate changes in gene expression. These reversible phosphorylation events are performed by a family of proteins known as kinases, whose activity in return is counteracted by another protein family called phosphatases.
Phosphorylation may result in conformational changes that trigger the activation or inactivation of an enzyme. The latter scenario is well documented for glycogen synthase kinase-3 beta (GSK3 beta), which upon phosphorylation by Protein Kinase B (PKB/AKT1) on serine 9 becomes inactivated (Doble and Woodgett 2003). In most cases, the interactions of kinases with their substrates are highly transient and therefore challenging to study.
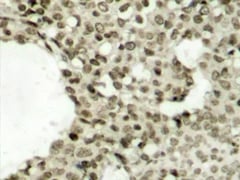
Immunohistochemical analysis of paraffin-embedded human breast carcinoma tissue using Rabbit anti STAT1 (pSer727) antibody.
View our Phospho-Specific PrecisionAb Antibody RangeIn addition to changes in protein activity levels, phosphorylated residues provide docking sites for adapter proteins or enable protein-protein interactions. Proteins containing forkhead (FHA), WD40 repeat and 14-3-3 domains bind to specific phosphoserine and phosphothreonine motifs (Mackintosh 2004). Binding of 14-3-3 proteins for instance mediates diverse processes ranging from eukaryotic autophagy and apoptosis pathways to plant metabolism (Pozuelo-Rubio 2012, Mackintosh 2004, Rosenquist 2003).
Proteins containing Src homology 2 (SH2) domains on the other hand bind to phosphotyrosine motifs (Schlessinger and Lemmon 2003). A well-studied group of SH2 domain containing proteins are Signal Transducers and Activators of Transcription (STATs), which upon tyrosine phosphorylation by Janus kinases (Jaks) form homo- or heterodimers. Once dimerized, STATs translocate from the cytoplasm to the nucleus, where they act as transcription factors (Rawlings et al. 2004). Changes in subcellular localization are a commonly observed phenomenon in response to phosphorylation.
Certain phosphorylation events are also characteristic of the activation of specific cell signaling pathways or cellular processes. For example, phosphorylation of the histone H2AX by the kinase ATM (Ataxia telangiectasia mutated) on serine 139, indicates the existence of DNA double strand breaks, the most toxic of all DNA lesions, which occurs in response to chemotherapy (Burma et al. 2001).
Bio-Rad offers a variety of antibodies for the detection of phosphoproteins by ELISA, immunofluorescence, immunohistochemistry and western blotting.
Our Phospho-Specific Antibody Range
| Description | Target | Format | Clone | Applications | Citations | Code |
|---|
- Growth factors
- EGF R resources
- Kinases
- mTOR signaling pathway
- Transcription factors
- Translation factors
- Phosphatases
- Phosphatases in cancer
- Phospho-Specific precisionAb antibodies
- Sumoylation mediated signaling
- Ubiquitination mediated signaling
- Tyrosine phosphorylation of EGF R
- Signal transduction antibodies
References
- Burma et al. (2001). ATM Phosphorylates Histone H2AX in Response to DNA Double-strand breaks. J Biol Chem 276, 42462-42467.
- Doble BW and Woodgett JR (2003). GSK-3: tricks of the trade for a multi-tasking kinase. Journal of Cell Science 116, 1175-1186.
- Mackintosh C (2004). Dynamic interactions between 14-3-3 proteins and phosphoproteins regulate diverse cellular processes. Biochem J 381, 329-342.
- Pozuelo-Rubio M (2012). 14-3-3 Proteins are Regulators of Autophagy. Cells 1, 754-773.
- Rawlings et al. (2004). The JAK/STAT signaling pathway. Journal of Cell Science 117, 1281-1283.
- Rosenquist M (2003). 14-3-3 proteins in apoptosis. Braz J Med Biol Res 36, 403-408.
- Schlessinger J and Lemmon MA (2003). SH2 and PTB domains in tyrosine kinase signaling. Sci STKE (191): RE12.