Article: Generation of Antibodies Against Self-Antigens Using HuCAL® Technology
-
Monoclonal Generation
-
Custom Recombinant Monoclonal Antibody Generation
-
Webinars, Videos and Technical Articles
- Webinar: Overcome the Challenges of PK Assay Development Using TrailBlazer Antibodies
- Webinar: Generation of SARS-CoV-2 antibodies in multiple formats within four weeks
- Webinar: Recombinant Antibodies with SpyTag Technology
- Webinar: Transform bioanalytical assays with TrailBlazer Antibodies
- Webinar: Control your critical antibody reagents and avoid assay failure
- Webinar: Improve your antibody drug development assays
- Webinar: Optimize your assays using recombinant antibodies selected for desired affinity
- Webinar: The making of recombinant anti-idiotypic antibodies for high performance in bioanalytical assays
- Webinar: Human recombinant antibodies as positive controls and calibrators
- Webinar: How to overcome assay challenges using custom recombinant antibodies
- Webinar: Generation of high affinity recombinant antibodies for application in immuno-MRM
- Video: Generating anti-idiotypic antibodies for bioanalytical assays
- Video: Best practices for characterization and QC of anti-idiotypic antibodies for bioanalysis
- Video: Generation of drug-target complex specific antibodies
- Video: Antibodies for CAR-T cell therapy development
- Article: Monitoring antibody immune responses against biotherapeutic drugs
- Article: Effective tools for drug monitoring assays
- Article: An accelerated approach to sensitive ADA assays
- Article: Isolation of enzyme active site-specific recombinant antibodies by guided selection
- Article: Biomarker Assay Development using Highly Specific Recombinant Antibodies
- Article: Recombinant antibodies as standards for immunodiagnostic assays
- Article: High affinity antibodies for peptide enrichment immuno-MRM
- Article: Generation of antibodies against self-antigens
- Article: Generation and characterization of drug-target complex-specific antibodies
- Article: Antibodies for CAR-T Cell Therapy Development
- Poster: Faster Generation of Anti-Drug Antibodies Using SpyTag Technology
- Poster: CAR T cell analysis with modular antibodies
- Poster: Drug-Target-Complex Specific Antibodies for Pharmacokinetic Analysis of Biotherapeutics
- Poster: Characterization of anti-idiotypic antibodies for high performance in bioanalytical assays
- Poster: Recombinant anti-idiotypic antibodies for antibody drug development
- Poster: Generation of recombinant antibodies for Bio-Plex assays
-
Webinars, Videos and Technical Articles
-
Custom Recombinant Monoclonal Antibody Generation
s
Simplified sourcing via Scientist.com
s
Custom antibody project inquiry form
A personal, no obligation quotation for a custom monoclonal antibody generation project
s
Contact our custom antibody specialists
Tel: +49 (0) 89 80 90 95 45
Fax: +49 (0) 89 80 90 95 50
Office: Bio-Rad AbD Serotec GmbH, Campus Neuried, Anna-Sigmund-Str. 5, 82061 Neuried, Germany
Cardioprotective Antibodies for a Murine Model of Myocardial Infarction
Human Combinatorial Antibody Library (HuCAL) technology was used to isolate a chimeric antibody specific to mannose-binding lectin-associated serine protease 2 (MASP-2) that inhibits the lectin pathway of the complement system in mice.
The generation of monoclonal antibodies against a murine protein through traditional methods of mouse immunization can result in difficulties eliciting a strong enough immune response against a self-antigen. HuCAL technology overcomes tolerance mechanisms and eliminates the need for animals; excellent antibody specificity can be achieved through ‘Guided Selection’. In this example, a human anti-rodent MASP-2 antibody was selected and then further engineered into a human/mouse IgG2a chimeric molecule, for in vivo studies in mice. This enabled scientists to carry out target validation studies in a multitude of disease models, including a mouse model of myocardial infarction, demonstrating the therapeutic potential of inhibiting MASP-2.
Monoclonal antibodies make ideal drugs due to their ability to bind with high affinity and specificity to their target. A large number of monoclonal antibody drugs have already been developed and licensed for a variety of different conditions.
Studies have demonstrated that the lectin complement pathway is a crucial mediator of injury following myocardial infarction (Panagiotou et al. 2018) and studies in a MASP-2 deficient mouse line, which cannot activate the lectin complement pathway, have shown significant reduction in post-acute myocardial infarction (Schwaeble et al. 2011), demonstrating the therapeutic potential of targeting this pathway. Creation of an antibody that inhibits MASP-2 for administration at a dose that is optimized to block lectin pathway activation and reduce lectin pathway-mediated inflammation following a cardiac event, could reduce infarct size and increase myocardial salvage.
A collaboration of scientists from academia and industry, including Dr James Clark, King’s College London (UK) and Dr Thomas Dudler, Omeros Corporation (USA), carried out a study using a HuCAL generated monoclonal antibody against MASP-2 to test feasibility of MASP-2 inhibition as an effective therapeutic intervention in a murine model of acute myocardial infarction (Clark et al. 2017).
Ischemic heart disease, including myocardial infarction (heart attack), is a leading cause of death worldwide. Myocardial infarction leads to irreversible necrosis of myocardial tissue due to ischemia, and the resulting inflammatory response triggered by the cardiac event is central to complications associated with the event and further disease progression.
MASP-2 is the initiating protease of the lectin complement pathway, a key pathway in the inflammatory response to tissue injury, and consequently central to the pathogenesis of inflammatory tissue injury. Inhibition of MASP-2, and subsequent down-regulation of the complement pathway, offers an opportunity to ameliorate the resulting infarction and increase myocardial salvage.
About HuCAL Technology
The structural diversity of the human antibody repertoire is represented in the HuCAL PLATINUM® library by seven heavy chain and six light chain variable region genes, which give rise to 42 master frameworks. Highly diverse genetic cassettes, encoding the complementarity determining regions (CDRs) of the antibody binding sites, are combined with these frameworks to create antibody genes that code for some 45 billion unique antibodies in Fab format (Knappik et al. 2000; Prassler et al. 2011).
The screening of the HuCAL library is performed in vitro enabling the successful selection of antibodies that it is not possible to generate by in vivo immunization of animals; such challenging targets include highly conserved or self-antigens, low immunogenic antigens and conformational variants. An additional benefit of in vitro selection is the direct access to the genetic information of the antibody, which enables a fast conversion of the original Fab antibody into a large number of different formats, including full length antibodies of different isotypes. As the sequence of any selected antibody is known, it is possible to reproduce the genes synthetically if needed. This sequence back up secures the future supply of the antibody, and recombinant production methods ensure there is a high level of consistency between batches.
HuCAL technology is proven and well published, and has been used by the Bio-Rad custom antibody team to generate antibodies for research and diagnostic applications since 2004.
Selection of Highly Specific Antibodies
The Bio-Rad team ‘panned’ the library for antibodies with specificity to recombinant rat MASP-2, which has high homology to the mouse protein. They used a technique called phage display blocking to identify and select phages and isolate the genes for antibodies with specificity for MASP-2 (Figure 1). Blocking was performed with milk and bovine serum albumin (BSA). Unbound and blocked phages were removed by extensive washing; the bound phages were eluted for further rounds of panning under the same blocking conditions. This ensured that only phages displaying antibodies that recognize MASP-2 were isolated for screening, sequencing, and later production in E. coli.
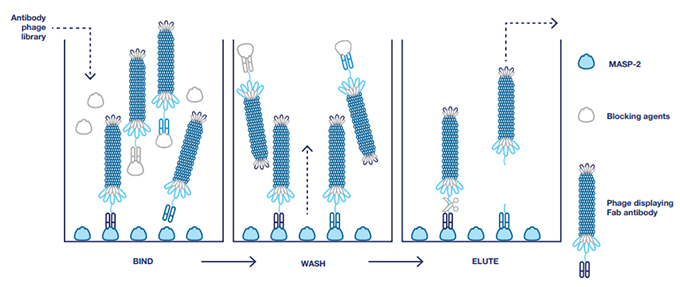
Fig. 1. Selection of antibodies with blocking. BSA and milk were used as blocking agents to remove nonspecific antibodies and ensure isolation of antibodies specific to MASP-2.
Antibody Reformatting for In Vivo Studies in Mice
After initial testing of antibodies in F(ab)2 format to select the antibody with the best inhibitory activity, the human Fab was subcloned into the pMORPH2_h/m_Ig vector series for human/mouse IgG2a. These vectors contain the mouse gamma 2a constant region, or the mouse lambda constant region. The resulting human/mouse chimeric molecule contained the human Fv comprising the antigen binding site fused to murine constant Ig2a and lambda regions, suitable for in vivo studies in mice.
In order to determine a dosing regimen for the infarction study, the anti-MASP-2 antibody, clone AbD04211, was administered in mice and lectin pathway inhibitory activity was assessed on a C4 deposition assay. A single dose of 1.0 mg/kg i.p was effective in inhibiting lectin pathway-dependent C4 deposition below the detection threshold of the assay for up to 1 week post-treatment (Figure 2).
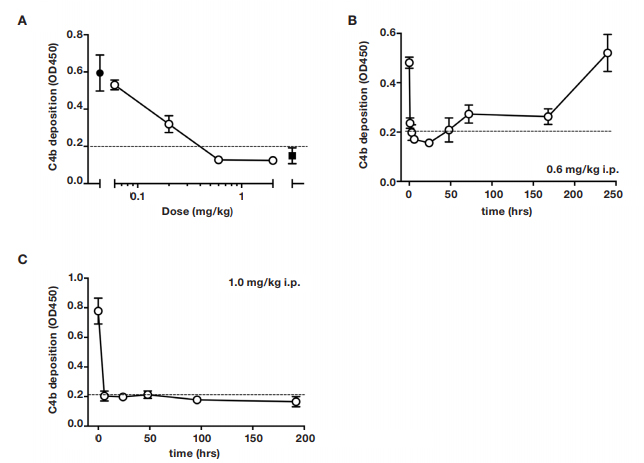
Fig. 2. Dose-response of lectin pathway (LP) inhibition following administration of anti-MASP-2 mAb (AbD04211) into C27BL/6 mice. (A) AbD04211 (0.06, 0.2, 0.6 or 2.0 mg/kg) or vehicle (PBS) was injected (i.p.) into mice and terminal plasma samples collected 6 hours after dosing. LP was assessed in 90% serum using C4b deposition onto mannan-coated plates as readout. Each data point represents
mean
±SD of three mice. C4 deposition in serum from mice dosed with vehicle (black-filled circle) and C4 deposition using the same serum supplemented with 50 nM of AbB04211 in vitro (black-filled square) are shown for reference. Time-course of LP ablation and recovery following administration (i.p.) of 0.6 mg/kg (B) or 1 mg/kg (C) AbD04211 into mice. Dashed lined shows baseline C4 deposition independent of LP (naive mouse serum supplemented with 50 nM of AbD04211 in vitro).
Anti-MASP-2 Antibodies Reduce Infarct Size
The team of researchers injected mice with anti-MASP-2 antibody or control prior to experimental infarction by ligation of the left anterior coronary artery for 30 minutes, resulting in ischemia, followed by 120 minutes reperfusion in vivo. Infarcts were significantly smaller in the anti-MASP-2 antibody treated group compared with the control isotype group and vehicle treated group (Figure 3).
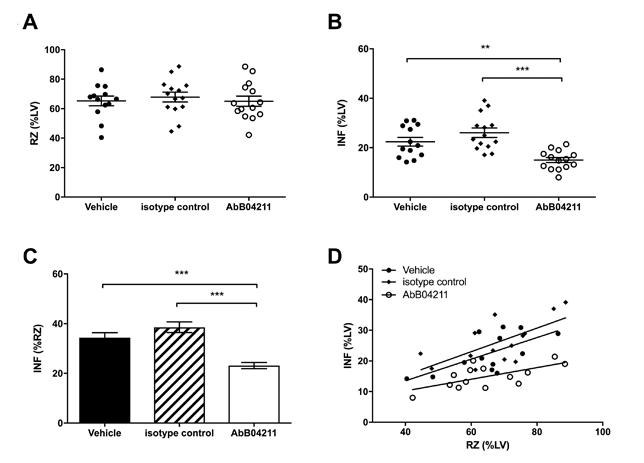
Fig. 3. Infarct and risk zone (RZ) assessments. Mice were subjected to 30 min
ischaemia
followed by 120 min reperfusion in vivo. RZ, infarct size (INF) and left ventricle (LV) size were measured by Evans blue and tetrazolium staining. (A) RZs determined in mice pretreated with vehicle (PBS), isotype control Ab or AbD04211; (B) INF as a percentage of LV mass and (C) INF as a percentage of RZ in the three groups. (D) INF versus RZ in all the experimental mice. Data are presented as
total
area at risk and INF as a percentage of LV volume. Results are mean ±SD of 13-15 independent experiments. **P<0.01; ***P<0.005 as indicated.
Conclusion
As demonstrated in this study, treatment with an anti-MASP-2 antibody blocking the lectin pathway of complement pathway, reduces the resulting infarction size and increases myocardial salvage.
This validation of MASP-2 as a feasible, druggable target provided important proof-of-concept data supporting the development of OMS721, a fully human monoclonal antibody against MASP-2 generated by the pharmaceutical company Omeros Corporation, independently of Bio-Rad and AbD04211. Omeros holds worldwide exclusive licenses to rights related to MASP-2, inhibitors targeting MASP-2, and the therapeutic applications for these inhibitors.
OMS721 is currently being tested in a number of clinical trials to evaluate its efficacy and safety in patients with disorders associated with lectin pathway activation. These diseases are characterized by significant tissue injury and pathology and include disorders such as thrombotic microangiopathies and complement-associated renal diseases.
The FDA has fast-tracked OMS721 for treatment of atypical hemolytic uremic syndrome (aHUS) and has granted OMS721 breakthrough therapy designation for the treatment of thrombotic microangiopathies and for the treatment of IgA nephropathy; the Phase 3 clinical trials for aHUS (NCT03205995) and IgA nephropathy are still ongoing.
References
- Clark JE et al. (2017). Cardioprotection by an anti-MASP-2 antibody in a murine model of myocardial infarction. Open Heart, 5, e000652.
- Knappik A et al. (2000). Fully synthetic human combinatorial antibody libraries (HuCAL) based on modular consensus frameworks and CDRs randomized with trinucleotides. J Mol Biol, 296, 57-86.
- Panagiotou A et al. (2018). The Lectin Pathway of Complement in Myocardial ischemia/Reperfusion injury — Review of its Significance and the Potential impact of Therapeutic interference by C1 esterase inhibitor. Front. Immunol.
- Prassler J et al. (2011). HuCAL PLATINUM, a synthetic Fab library optimized for sequence diversity and superior performance in mammalian expression systems. J Mol Biol, 413, 261-278.
- Schwaeble et al. (2011) Targeting of mannan-binding lectin-associated serine protease-2 confers protection from myocardial and gastrointestinal ischemia/reperfusion injury. Proc Natl Acad Sci U S A, 108, 7523-8
- Figures 2 and 3 are reproduced from Open Heart, Clark JE, Dudler T, Marber MS, et al, 5, e000652. doi: 10.1136/openhrt-2017-000652, 2018, with permission from BMJ Publishing Group Ltd.






