Article: Effective Tools for Drug Monitoring Assays
-
Monoclonal Generation
-
Custom Recombinant Monoclonal Antibody Generation
-
Webinars, Videos and Technical Articles
- Webinar: Overcome the Challenges of PK Assay Development Using TrailBlazer Antibodies
- Webinar: Generation of SARS-CoV-2 antibodies in multiple formats within four weeks
- Webinar: Recombinant Antibodies with SpyTag Technology
- Webinar: Transform bioanalytical assays with TrailBlazer Antibodies
- Webinar: Control your critical antibody reagents and avoid assay failure
- Webinar: Improve your antibody drug development assays
- Webinar: Optimize your assays using recombinant antibodies selected for desired affinity
- Webinar: The making of recombinant anti-idiotypic antibodies for high performance in bioanalytical assays
- Webinar: Human recombinant antibodies as positive controls and calibrators
- Webinar: How to overcome assay challenges using custom recombinant antibodies
- Webinar: Generation of high affinity recombinant antibodies for application in immuno-MRM
- Video: Generating anti-idiotypic antibodies for bioanalytical assays
- Video: Best practices for characterization and QC of anti-idiotypic antibodies for bioanalysis
- Video: Generation of drug-target complex specific antibodies
- Video: Antibodies for CAR-T cell therapy development
- Article: Monitoring antibody immune responses against biotherapeutic drugs
- Article: Effective tools for drug monitoring assays
- Article: An accelerated approach to sensitive ADA assays
- Article: Isolation of enzyme active site-specific recombinant antibodies by guided selection
- Article: Biomarker Assay Development using Highly Specific Recombinant Antibodies
- Article: Recombinant antibodies as standards for immunodiagnostic assays
- Article: High affinity antibodies for peptide enrichment immuno-MRM
- Article: Generation of antibodies against self-antigens
- Article: Generation and characterization of drug-target complex-specific antibodies
- Article: Antibodies for CAR-T Cell Therapy Development
- Poster: Faster Generation of Anti-Drug Antibodies Using SpyTag Technology
- Poster: CAR T cell analysis with modular antibodies
- Poster: Drug-Target-Complex Specific Antibodies for Pharmacokinetic Analysis of Biotherapeutics
- Poster: Characterization of anti-idiotypic antibodies for high performance in bioanalytical assays
- Poster: Recombinant anti-idiotypic antibodies for antibody drug development
- Poster: Generation of recombinant antibodies for Bio-Plex assays
-
Webinars, Videos and Technical Articles
-
Custom Recombinant Monoclonal Antibody Generation
s
Simplified sourcing via Scientist.com
s
Custom antibody project inquiry form
A personal, no obligation quotation for a custom monoclonal antibody generation project
s
Contact our custom antibody specialists
Tel: +49 (0) 89 80 90 95 45
Fax: +49 (0) 89 80 90 95 50
Office: Bio-Rad AbD Serotec GmbH, Campus Neuried, Anna-Sigmund-Str. 5, 82061 Neuried, Germany
Scientists Validate Recombinant Monoclonal Antibodies for PK and Immunogenicity Assays
Sensitive and reproducible pharmacokinetic (PK) and immunogenicity assays are required as part of the complex and lengthy development process for biotherapeutic proteins. These assays are included in a range of approaches applied to understand the nature and properties of the drug as well as the induction of anti-drug antibodies (ADA) against the target, which can cause adverse events and loss of efficacy.
There are particular challenges to developing drug monitoring assays when the biotherapeutic is itself a human or humanized monoclonal antibody, due to the excess of human antibodies in patient serum. Consequently, PK assays to monitor the drug in human sera require highly specific detection reagents that bind to the drug, but not to the similar immunoglobulin molecules. A well designed ADA assay requires a highly specific reference antibody against which to measure an anti-drug response. For the clinical phases of development it should ideally be one or more fully human antibodies with varying affinities and/or varying immunoglobulin subclasses in order to reflect the natural immune response. Anti-idiotypic antibodies, targeting the individual antibody drug complementarity determining regions (CDR) are recognized as optimal components for such PK and ADA assays.
Tornetta and colleagues at Janssen Biotech, Inc. formerly Centocor, (Tornetta et al. 2007) set out to validate the use of fully human anti-idiotypic antibodies generated using HuCAL® technology. They compare their performance with antibodies developed using the traditional murine hybridoma method and with polyclonal sera from immunized primates. The target antigens were engineered human antibodies, directed against human IL-13 (IgG1/λ) and human IL-6 (IgG1/κ). The HuCAL antibody library was panned against biotinylated versions of these two antigens in the presence of pooled human serum to eliminate binding to shared regions in human IgG1 and cross-reactivity with other human serum proteins. A framework matched control monoclonal antibody was also included in the strategy for counter-selection, to guide antibody specificity to the CDRs of the target antigens.
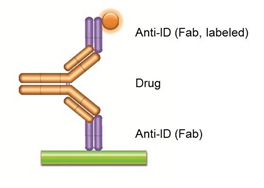
Fig. 1. (a) PK Assay Anti-idiotypic Antibody Bridging Assay
Using the same or two different anti-idiotypic antibodies (anti-IDs) in this assay, the drug to be measured bridges the capture and the detection reagents due to its bivalent nature. A highly specific anti-idiotypic antibody (purple, Fab antibody) is immobilized at low concentration and captures the drug (gold antibody) in the serum sample. The bound drug is then quantified with a second labeled anti-idiotypic antibody that binds to the free idiotope of the drug
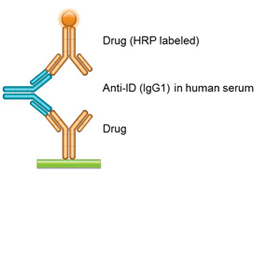
(b) ADA Assay Anti-idiotypic Antibody Bridging Assay
In this assay the anti-idiotypic antibody is used as a surrogate for anti-drug antibodies that might be developed by a patient. The drug (gold antibody) is immobilized, and the anti-idiotypic antibody (blue IgG antibody) is added in increasing concentrations. The bound anti-idiotypic antibody is detected by adding labeled drug. The response curve depends on the affinity of the anti-drug antibody. The bridging assay can be established using anti-idiotypic antibodies with a range of affinities, as well as different immunoglobulin subclasses to reflect the natural immune response.
The Fab antibodies generated were tested as capture and detection antibodies in ELISAs and antigen competition assays to assess their suitability as reagents for PK studies. The best capture and detection Fab antibodies were identified and used to establish highly specific sandwich ELISAs for both target antibodies (Figure 1A). The affinities of the Fab antibodies, determined from measurement of the association and dissociation rate constants, were between 0.08 and 6.5 nM. If needed, the affinity of the HuCAL Fab could be further increased by affinity maturation to enhance the sensitivity of PK assays
For the anti-human IL-13 project, selected anti-idiotypic Fab antibodies were converted into a fully human IgG format in order to evaluate their potential as surrogate positive controls in ADA assays (Figure 1B). Bridging ELISAs were developed comparing the phage-derived antibodies, mouse monoclonal antibodies, and monkey polyclonal serum. The experiments confirmed specificity of the phage-derived antibodies to the CDRs and no binding to endogenous human serum antibodies and proteins. The phage-derived antibodies more closely mimicked the performance of the primate anti-human immune serum in bridging ELISA assays than the murine anti-idiotypic antibodies, which gave misleading results, attributed to cross-reactivity with other human immunoglobulins in the sample matrix. Moreover, having a fully human reference antibody already available at the preclinical stage of development confers the added advantage of not having to revalidate assays with different reagents at the clinical development stage.
In the researchers hands the panel of fully human, anti-idiotypic antibodies satisfied the necessary criteria for highly specific reagents for their preclinical and clinical assays. In particular, for the ADA assays, the in vitro produced antibodies overcame the limitations of antibodies derived using the traditional method of murine hybridoma generation, with the added benefit of a secure, consistent and long-term supply.
References
-
Tornetta, M. et al. 2007. Isolation of human anti-idiotypic antibodies by phage display for clinical immune response assays.
J Immunol Methods 328(1-2):34-44.






