Mini-review: The Role of Immune Checkpoints in Immunity and Cancer
Immune checkpoints are regulators of immune activation. They play a key role in maintaining immune homeostasis and preventing autoimmunity. In cancer, immune checkpoint mechanisms are often activated to suppress the nascent anti-tumor immune response. This has led to the development of several checkpoint inhibitor antibody drugs that are currently being tested in clinical trials or have been approved for a number of cancers. This mini-review provides an overview of the mechanisms of action of major immune checkpoint molecules as well as highlights checkpoint inhibitor antibodies in clinical development.
Function of immune checkpoints in regulating immunity
Maintaining immune homeostasis is critical for host survival. Overt or uncontrolled immune responses to pathogens or mutated/overexpressed self-antigens can cause inflammatory tissue damage and autoimmune diseases. To prevent this, the breadth and magnitude of the immune response is regulated by a balance between co-stimulatory and inhibitory signals. These signals are collectively referred to as immune checkpoints, which are necessary for maintaining self-tolerance and protecting the host from tissue damage.
Activated T cells are primary mediators of immune effector functions and as such, they express multiple co-inhibitory receptors such as lymphocyte-activation gene 3 (LAG-3), programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). These immune checkpoint molecules have been shown to modulate T cell responses to self proteins as well as to chronic infections and tumor antigens (Pardoll 2012). Notably, the pathways utilized by these checkpoint proteins are unique and non-redundant (Nirschl and Drake 2013). This demonstrates the important role of immune checkpoints in regulating immune homeostasis, and provides a rationale for targeting multiple immune checkpoints to enhance anti-tumor immunity (discussed in section 2).
Below we discuss the mechanism of action of major immune checkpoint proteins.
CTLA-4
Of the immune checkpoint proteins identified to date, the mechanisms by which CTLA-4 inhibits T cell function is the best understood. CTLA-4 is a transmembrane glycoprotein that is a homolog of the immune co-stimulatory protein CD28. CTLA-4 plays a key role in developing peripheral tolerance to self proteins by neutralizing the function of CD28 (Rudd et al. 2009). Following engagement of the T cell receptor (TCR) with cognate antigen, CD28 provides the second signal for T cell activation by binding to CD80 (B7-1) and CD86 (B7-2) proteins on antigen presenting cells (APCs). CTLA-4 binds these B7 proteins with approximately 20 times greater affinity and can therefore outcompete CD28 for binding (Kim et al. 2015).
To induce its inhibitory effects on T cells, CTLA-4 utilizes both signaling and non-signaling mechanisms. CTLA-4 becomes phosphorylated after binding B7 proteins and subsequently binds phosphoinositide 3-kinase (PI3K) (Rudd et al. 2009). This leads to activation of the phosphatases SHP2 (also known as PTPN11) and PP2A (Marengere et al. 1996, Chuang et al. 2000).
The association of CTLA-4 with SHP2 leads to dephosphorylation of the CD3ζ chain, thereby limiting the signaling potential of the TCR (Pardoll 2012). CTLA-4 recruitment of PP2A results in decreased downstream Akt phosphorylation, which diminishes T cell activation signaling initiated by TCR engagement with antigen (Parry et al. 2005). Activation of these phosphatases by CTLA-4 also counteracts the kinase signals induced by the TCR and CD28 (Pardoll 2012). CTLA-4 also exerts its inhibitory function through signal independent mechanisms. CTLA-4 can actively remove CD80 and CD86 from the APC surface through the process of transendocytosis (Qureshi et al. 2011).
Inhibition of CTLA-4 enhances immune responses that are dependent on CD4+ T helper cells, indicating that its major physiological role primarily involves this T cell subset (Pardoll 2012). In addition, CTLA-4 blockade leads to inhibition of the immunosuppressive function of Tregulatory cells (Tregs), as these cells constitutively express this immune checkpoint protein (Pardoll 2012).
The significant role of CTLA-4 in immunity is clearly demonstrated in Ctla4-knockout mice, which are moribund by 3-4 weeks old and exhibit severe pancreatitis, myocarditis and T cell infiltration in the liver, heart, lung and pancreas (Tivol et al. 1995, Waterhouse et al. 1995).
PD-1/programmed death ligand 1 or 2 (PD-L1 or PD-L2)
In contrast to CTLA-4, PD-1 predominantly regulates effector T cell activity within tissues and tumors as opposed to regulating T cell activation in lymphoid organs (Pardoll 2012). While CTLA-4 mainly affects naïve T cells, PD-1 is primarily expressed on mature T cells in peripheral tissues and the tumor microenvironment. It is also expressed on other non-T cell subsets including B cells, professional APCs and natural killer (NK) cells.
PD-1 signaling is mediated through interaction with its ligands PD-L1 (also known as B7-H1 and CD274) and PD-L2 (also known as B7-DC and CD273) (Nirschl and Drake 2013). Studies also indicate that PD-L1 interacts with the CTLA-4 and CD28 ligand, CD80, to inhibit T cell proliferation (Butte et al. 2007). In this case, CD80 acts as a receptor and delivers inhibitory signals when engaged with PD-L1 (Park et al. 2010).
Interaction of PD-1 with any of its ligands leads to dephosphorylation and inactivation of the T cell kinase ZAP70, and the recruitment of SHP2. SHP2 directly dephosphorylates PI3K, which inhibits downstream activation of Akt (Kim et al. 2015). This ultimately leads to decreased production of inflammatory cytokines and cell survival proteins such as Bcl-xL (Parry et al. 2005). Similar to CTLA-4, PD-1 is highly expressed on Tregs, and enhances their proliferation and suppressive activity upon ligand binding (Pardoll 2012).
These findings demonstrate the unique and complex mechanisms of action of PD-1. Accordingly, PD-1 knockout mice demonstrate autoimmunity (Nishimura et al. 2001). Specifically, they have elevated levels of IgG2b and IgA and develop mild lupus-like autoimmunity and dilated cardiomyopathy (Nishimura et al. 2001). These disease phenotypes however depend on the mouse strain used and occur later in life. The PD-1 knockout autoimmune effects are also less severe than those observed in CTLA-4 knockout animals (Sharpe et al. 2007).
LAG-3
LAG-3 (also known as CD223) is a homolog of CD4, cloned over 25 years ago (Triebel et al. 1990). Similar to CD4, the only known ligand for LAG-3 is the major histocompatibility complex (MHC) class II molecule. Like CTLA-4 and PD-1, LAG-3 is also critical for dampening overt T cell immune responses. LAG-3 specifically inhibits CD8+ effector T cell functions and can enhance the suppressive activity of Tregs (Grosso et al. 2007, Huang et al. 2004). Under inflammatory conditions, LAG-3 is expressed in vivo on the surface of activated CD4+, CD8+ and NK cells (Kim et al. 2015). LAG-3 expression is also upregulated on anergic T cells, and antibody blockade of LAG-3 can reverse this anergic state to a certain degree (Pardoll 2012). Since PD-1 and LAG-3 are commonly co-expressed on anergic T cells, dual blockade of these receptors resulted in reversed anergy in a chronic infection setting (Blackburn et al. 2009).
It is unclear whether PD-1 and LAG-3 share similar mechanisms of action. In fact, the molecular pathways mediating LAG-3 signaling are largely unknown. However, studies indicate that the unique intracellular KIEELE domain is required for its function (Workman and Vignali 2003). LAG-3 knockout mice exhibit less severe autoimmune effects than CTLA-4 and PD-1 knockout animals (Miyazaki et al. 1996). This indicates that LAG-3 may play a more subtle role than these key immune checkpoint molecules in regulating T cell function (Nirschl and Drake 2013).
T cell immunoglobulin mucin 3 (TIM-3)
TIM-3 was discovered in 2002 as a marker for interferon-gamma (IFN-γ) producing CD8+ T cells (Kim et al. 2015). It is a glycoprotein that has extracellular immunoglobulin and mucin domains. TIM-3 is expressed on a number of cells such as activated T cells as well as tissues such as the liver, small intestine, thymus, kidney, spleen, lung, muscle and brain (Wada and Kanwar 1997). The most prominent ligand for TIM-3 is galectin. However, other ligands have been identified such as phosphatidyl serine and high mobility group box 1 (HMGB1) (Zhu et al. 2005, Gorman and Colgan 2014).
Signaling through TIM-3 is dependent on phosphorylation at Y265 by inducible T cell kinase (van de Weyer et al. 2006). Studies in autoimmune models also show that the cytoplasmic protein Bat3 functions as an adapter protein to modulate cell proliferation. In this context, Bat3 binds TIM-3 at rest and protects the T cell from TIM-3 signaling. However, when TIM-3 binds to galectin 9, Bat3 dissociates from TIM-3, which leads to decreased production of IFN-γ and reduced T cell proliferation (Rangachari et al. 2012).
Killer immunoglobulin-like receptors (KIRs)
KIRs are a broad category of receptors that primarily bind MHC I molecules and inhibit NK cell function (Lanier 2008). In addition to NK cells, these receptors are also expressed on T cells (specifically tumor-associated cytotoxic T cells) and APCs (Mingari et al. 2005). However, their inhibitory role on T cells and APCs is less well studied (Pardoll 2012).
KIRs generally contain 2-3 immunoglobulin (Ig) ectodomains and cytoplasmic tails of various lengths (Long et al. 1997). However, they can be separated into two distinct subclasses based on structure and function. Some KIRs are activating and have truncated cytoplasmic tails and a positively charged residue in their transmembrane domain. In contrast, others are type II transmembrane receptors containing two immune receptor tyrosine-based inhibitory motifs (ITIMs), which facilitate inhibitory signaling. ITIMs mediate downstream signaling leading to negative regulation of NK cell function, reduction of NK cell mediated lysis and NK tolerance to self (Kim et al. 2015).
KIRs induce NK cell tolerance through a process called licensing. During this process, the KIR recognizes a self MHC I molecule and prevents NK cell activation against self-tissue and auto-antigens (Yu et al. 2009). There are more than 20 KIRs, with many demonstrating specificity for subsets of human leukocyte antigens (HLAs) and allele specificity.
4-1BB
The 4-1BB receptor (also known as CD137) belongs to the tumor necrosis factor receptor (TNFR) superfamily. It is expressed on stimulated CD4+ and CD8+ T cells, activated NK cells, neutrophils and dendritic cells (Kim et al. 2015). It is a type II transmembrane glycoprotein that binds the 4-1BB ligand expressed on activated macrophages and B cells (Kim et al. 2015). In contrast to the other immune checkpoint molecules previously discussed, 4-1BB is an activating checkpoint. Upon ligation of the 4-1BB receptor, NF-κB, c-Jun and p38 signaling pathways become activated (Cannons et al. 2000). This ultimately promotes survival and pro-inflammatory pathways. In CD8+ T cells, 4-1BB receptor activation induces survival by upregulating the expression of anti-apoptotic genes BcL-xL and Bfl-1 (Lee et al. 2002). The main role of 4-1BB therefore is to boost the immune response.
Glucocorticoid-induced TNFR family related gene (GITR)
Similar to the 4-1BB receptor, GITR also belongs to the TNFR superfamily. It was initially identified as a marker of Tregs; however it is now known to also be constitutively expressed on effector CD4+ and CD8+ T cells (McHugh et al. 2002). The human ligand for GITR (GITRL) is constitutively expressed on APCs in secondary lymphoid organs and on non-lymphoid tissues (Kim et al. 2015). Once GITR engages with its ligand GITRL, the downstream signaling ultimately results in attenuation of Treg responses and enhancement of effector T cell responses (Kim et al. 2015). Therefore, like 4-1BB, GITR is also an activation immune checkpoint that enhances host immune responses. However, studies show that overexpression of GITR or experimental agonism of the receptor is associated with autoimmunity and inflammatory indications such as asthma and post-stroke states (Kohm et al. 2004, Patel et al. 2005, Takata et al. 2012).
The table below summarizes the features and signaling pathways of the major immune checkpoint molecules.
Table 1. Features and mechanisms of major immune checkpoint molecules.
Immune Checkpoint Protein |
Synonym |
Cellular Expression |
Ligand |
Mechanism of Action |
|---|---|---|---|---|
|
(immune suppressive checkpoint) |
CD152 |
Mostly naïve CD4+ and CD8+ T cells, Tregs |
CD80 (B7-1) or CD86 (B7-2) |
|
|
(immune suppressive checkpoint) |
CD279 |
Activated T cells in peripheral tissue, B cells, professional APCs, NK cells |
PD-L1 (CD274, B7-H1) and PD-L2 (CD273, B7-DC) |
|
|
(immune suppressive checkpoint) |
CD223 |
Activated T cells, NK cells |
MHC class II |
|
|
(immune suppressive checkpoint) |
HAVcr2 |
Activated T cells and tissues such as liver, small intestine, thymus, spleen, lung muscle, brain tissue |
Mainly galectin-9, as well as phosphatidylserine and HMGB1 |
|
|
(immune suppressive checkpoint) |
CD158 |
Mainly NK cells but also APCs and tumor associated CTLS |
MHC class I molecules (HLAs) |
|
|
(immune activating checkpoint) |
CD137 |
Activated CD4+ and CD8+ T cells, Tregs, activated NK cells, DCs, neutrophils |
4-1BB-L |
|
|
GITR (immune activating checkpoint) |
CD357 |
Tregs, activated CD4+ and CD8+ T cells |
GITRL |
|
Abbreviations: APCs, antigen presenting cells; CTLs, cytotoxic T lymphocytes; DCs, dendritic cells, CTLA-4, cytotoxic T lymphocyte-associated antigen 4 (CTLA-4); GITR, glucocorticoid-induced tumor necrosis factor family related gene; GITRL, glucocorticoid-induced tumor necrosis factor family related gene ligand; HLAs, human leukocyte antigens; HMGB1, high mobility group box 1; KIRs, killer immunoglobulin-like receptors; LAG-3, lymphocyte-activation gene 3; MHC, major histocompatibility complex; NF-κB, nuclear factor kappa B; NK cells, natural killer cells; PD-1, programmed cell death protein 1; PD-L1, programmed death 1 ligand 1; PI3K, phosphatidyl inositol 3 kinase; TIM-3, T cell immunoglobulin mucin; TNF, tumor necrosis factor; Tregs, T regulatory cells.
Blockade of immune checkpoints for cancer therapy
Cancer growth is partly mediated by immune suppression induced by cancers. Studies have demonstrated that tumors can activate suppressive immune checkpoint pathways in order to diminish the immune response to the tumor (Finn 2012). Scientists therefore investigated whether blockade of key immune checkpoint pathways could induce effective anti-tumor immunity. Initial preclinical research indicated that antibody blockade of the immune checkpoint molecule CTLA-4 resulted in successful anti-tumor immune responses in murine cancer models (Leach et al. 1996, van Elsas et al. 1999). Based on these findings, CTLA-4 was the first immune checkpoint molecule to be clinically targeted. This was then followed by antibodies targeting the PD-1/PD-L1 pathway. To date, the most advanced agents in clinical trials are those targeting CTLA-4 and PD-1/PD-L1 (Figure 1). Below we discuss the current inhibitor antibodies in clinical development.
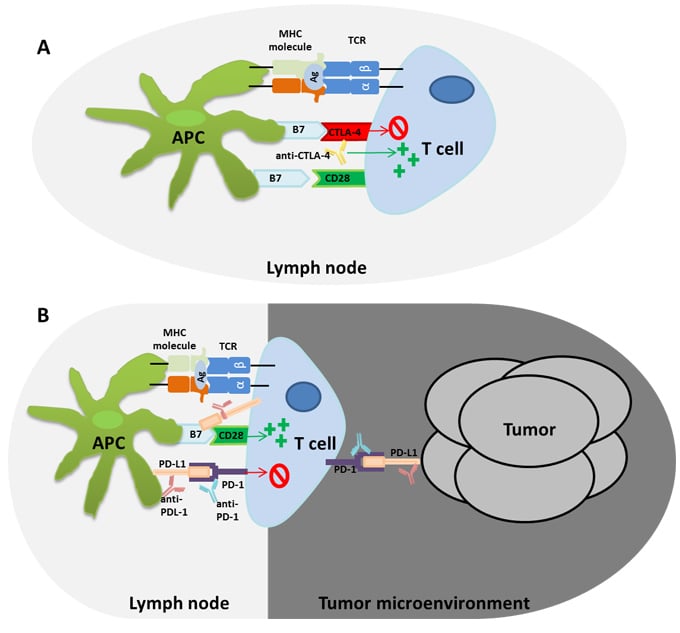
Fig.1. CTLA-4 and PD-1/PD-L1 immune regulatory pathways affected by immune checkpoint antibody drugs. Adapted from Postow et al. 2015. A) The immune checkpoint molecule cytotoxic T lymphocyte associated antigen 4 (CTLA-4) outcompetes CD28 to bind the B7 ligand on antigen presenting cells (APCs). This leads to inhibition of T cell activation. However, the anti-CTLA-4 antibody can block this interaction resulting in activated antigen specific T cells that can induce anti-tumor immunity. B) The programmed death ligand 1 (PD-L1) and programmed death 1 (PD-1) immune checkpoint pathway inhibit T cell responses in cancer. Inhibition of PD-1 or PD-L1 using inhibitor antibody drugs has been shown to induce significant anti-tumor immune responses.
Inhibitor antibodies targeting the CTLA-4 immune checkpoint
Ipilimumab was the first anti-CTLA-4 drug developed. It is a fully humanized Ig G1 kappa monoclonal antibody that antagonizes CTLA-4 and inhibits ligand binding (Morse 2005). Two phase III studies involving patients with advanced melanoma showed that ipilimumab improved overall survival by several months. This led to its approval for the treatment of melanoma by the United States Food and Drug Administration (FDA) and the European Medicines Agency (Hodi et al. 2010, Robert et al. 2011). Pooled analysis of data from ipilimumab trials demonstrates that approximately 20% of patients will have long term survival of at least 3 years after ipilimumab therapy (Postow et al. 2015). Ipilimumab is still currently being evaluated for application in other indications, with significant attention to effective combination strategies with other checkpoint inhibitor antibodies.
The second anti-CTLA-4 antibody currently being evaluated in clinical trials is tremelimumab, a fully human IgG2 monoclonal antibody. It is being investigated as a monotherapy or in combination with durvalumab (a PD-L1 targeted antibody) in non-small cell lung (NSCLC), head and neck, gastric, pancreatic, blood cancers and hepatocellular carcinoma. It has also been tested as a potential treatment for malignant mesothelioma and advanced melanoma.
A phase III clinical trial comparing tremelimumab treatment to standard-of-care chemotherapy (dacarbazine/temozolomide) in patients with advanced melanoma demonstrated disappointing results. Although some patients experienced a durable response, tremelimumab did not demonstrate significant survival advantage over standard-of-care chemotherapy treatment (Ribas et al. 2013). Recently, a phase IIb trial assessing the efficacy of tremelimumab as a monotherapy in mesothelioma also reported dismal results, as the trial failed to meet its primary endpoint of improving overall survival (AstraZeneca 2016). Another phase Ib study investigating the anti-tumor activity of tremelimumab and durvalumab in locally advanced or metastatic NSCLC patients demonstrated significant antitumor activity. Notably, the anti-tumor effect of the combination therapy was independent of PD-L1 status, and phase III studies are now on-going (Antonia et al. 2016). Further research is also being conducted to identify biomarkers for predicting which patients are likely to respond to anti-CTLA-4 therapies.
Inhibitor antibodies targeting the PD-1/PD-L1 immune checkpoint
Owing to the success of ipilimumab, several anti-PD-1 and anti-PD-L1 antibodies have been developed. Those that target PD-1 include nivolumab, pembrolizumab, and pidilizumab. PD-L1 targeted antibodies are atezolizumab (MPDL3280A), durvalumab (MEDI4736), BMS-936559 and MSB0010718C. In addition, a PD-L2 fusion protein that depletes PD-1 positive T cells is also under clinical development (Infante et al. 2013).
The results observed in clinical studies targeting the PD-1/PD-L1 pathway have been encouraging since higher response rates were reported than in CTLA-4 blockade studies (Sharon et al. 2014). Furthermore, PD-1/PD-L1 blockade generally results in less severe adverse effects, although fatal pneumonitis was observed in 1% of patients in an anti-PD-1 antibody clinical trial (Topalian et al. 2012).
A phase III study comparing nivolumab treatment to standard chemotherapy (dacarbazine or carboplatin/paclitaxel) involving melanoma patients who did not respond to ipilimumab treatment demonstrated a 32% overall response rate with nivolumab compared to only 11% with chemotherapy alone (Weber et al. 2015).
Pembrolizumab also demonstrated significant efficacy in clinical trials, leading to its approval by the FDA for treating patients with melanoma who were previously treated with ipilimumab and, if relevant, a BRAF inhibitor (Robert et al. 2014). Pidilizumab on the other hand is primarily being evaluated in hematological cancers and has demonstrated favorable responses as a monotherapy and in combination with other therapies such as rituximab (Berger et al. 2008, Armand et al. 2013, Westin et al. 2014).
Targeting PD-L1 has also demonstrated impressive results in the clinic. Phase II and III studies involving atezolizumab demonstrated consistent results in patients with NSCLC (Fehrenbacher et al. 2016, Rittmeyer A et al. 2017). Compared to docetaxel chemotherapy, treatment with atezolizumab resulted in a 4.2 month and 2.9 month improvement in overall survival in the phase II and III trials, respectively. This led to the FDA approval of atezolizumab in October 2016 for the treatment of patients with metastatic NSCLC whose disease progressed during or following platinum-containing chemotherapy.
Durvalumab is currently being tested in clinical trials as a monotherapy for metastatic urothelial cancer, and has demonstrated a manageable safety profile and significant clinical activity (Massard et al. 2016).
As the inhibitor antibodies discussed above move through clinical development and to patients, there is likely to be further opportunities to improve their effect. Particularly, more research is now being conducted to evaluate combination therapies focusing on the complementary mechanisms of action of these antibody drugs.
References
- Antonia S et al. (2016). Safety and antitumor activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol 17, 299-308.
- Armand P et al. (2013). Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse-large B-cell lymphoma: Results of an international phase II trial. J Clin Oncol 31, 4199-4206.
- Berger R et al. (2008). Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res 14, 3044-3051.
- Blackburn SD et al. (2009). Coregulation of CD8+ T cell exhaustion during chronic viral infection by multiple inhibitory receptors. Nat Immunol 10, 29-37.
- Butte MJ et al. (2007). PD-L1 interacts specifically with B7-1 to inhibit T cell proliferation. Immunity 27, 111-122.
- Cannons JL et al. (2000). Role of TNF receptor-associated factor 2 and p38 mitogen-activated protein kinase activation during 4-1BB-dependent immune response. J Immunol 165, 6193-6204.
- Chuang E et al. (2000). The CD28 and CTLA-4 receptors associate with the serine/threonine phosphatase PP2A. Immunity 13, 313-322.
- Fehrenbacher L et al. (2016). Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387, 1837-1846.
- Finn OJ (2012). Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol 23, viii6-viii9.
- Gorman JV and Colgan JD (2014). Regulation of T cell responses by the receptor molecule Tim-3. Immunol Res 59, 56-65.
- Grosso JF et al. (2007). LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest 117, 3383-3392.
- Hodi FS et al. (2010). Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363, 711-723.
- Huang CT et al. (2004). Role of LAG-3 in regulatory T cells. Immunity 21, 503-513.
- Infante JR et al. (2013). Clinical and pharmacodynamic (PD) results of a phase I trial with AMP-224 (B7-DC Fc) that binds to the PD-1 receptor. J Clin Oncol 31(suppl; abstr 3044).
- Kim ES et al. (2015). Immune checkpoint modulators: An emerging antiglioma armamentarium. J Immunol Res article ID: 4683607.
- Kohm AP et al. (2004). Ligation of the glucocorticoid-induced TNF receptor enhances autoreactive CD4+ T cell activation and experimental autoimmune encephalomyelitis. J Immunol 172, 4686-4690.
- Lanier LL (2008). Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol 9, 495-502.
- Leach DR et al. (1996). Enhancement of antitumor immunity by CTLA-4 blockade. Science 271, 1734-1736.
- Lee HW et al. (2002). 4-1BB promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xL and Bfl-1. J Immunol 169, 4882-4888.
- Long EO et al. (1997). Killer cell inhibitory receptors: diversity, specificity, and function. Immunol Rev 155, 135-144.
- Marengere LE et al. (1996). Regulation of T cell receptor signaling by tyrosine phosphatase SYP association with CTLA-4. Science 272, 1170-1173.
- Massard C et al. (2016). Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol 10, 3119-3125.
- McHugh RS et al. (2002). CD4+CD25+ immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity 16, 311-323.
- Mingari MC et al. (2005). Human cytolytic T lymphocytes expressing HLA class I inhibitory receptors. Curr Opin Immunol 17, 312-319.
- Miyazaki T et al. (1996). LAG-3 is not responsible for selecting T helper cells in CD4-deficient mice. Int Immunol 8, 725-729.
- Morse MA (2005). Technology evaluation: ipilimumab, Medarex/Bristol-Myers Squibb. Curr Opin Mol Ther 7, 588-597.
- Nirschl CJ and Drake CG (2013). Molecular pathways: Co-expression of immune checkpoint molecules: Signaling pathways and implications for cancer immunotherapy. Clin Cancer Res 19, 4917-4924.
- Nishimura H et al. (2001). Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291, 319-322.
- Pardoll DM (2012). The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12, 252-264.
- Park JJ et al. (2010). B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood 116, 1291-1298.
- Parry RV et al. (2005). CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 25, 9543-9553.
- Patel M et al. (2005). Glucocorticoid-induced TNFR family-related protein (GITR) activation exacerbates murine asthma and collagen-induced arthritis. Eur J Immunol 35, 3581-3590.
- Postow MA et al. (2015). Immune checkpoint blockade in cancer therapy. J Clin Oncol 33, 1974-1982.
- Press Statement by AstraZeneca (2016). AstraZeneca reports top-line result of tremelimumab monotherapy trial in mesothelioma. [online] Available at: https://www.astrazeneca.com/media-centre/press-releases/2016/astrazeneca-reports-top-line-result-of-tremelimumab-monotherapy-trial-in-mesothelioma-29022016.html Accessed 24 February 2017.
- Qureshi OS et al. (2011). Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science 332, 600-603.
- Rangachari M et al. (2012). Bat3 protects T cell responses by repressing Tim-3 mediated exhaustion and death. Nat Med 18, 1394-1400.
- Ribas A et al. (2013). Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma J Clin Oncol 31, 616-622.
- Rittmeyer A et al. (2017). Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389, 255-265.
- Robert C et al. (2011). Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 364, 2517-2526.
- Robert C et al. (2014). Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomised dose-comparison cohort of a phase 1 trial. Lancet 384, 1109-1117.
- Rudd CE et al. (2009). CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev 229, 12-26.
- Sharon E et al. (2014). Immune checkpoint inhibitors in clinical trials. Chin J Cancer 33, 434-444.
- Sharpe AH et al. (2007). The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol 8, 239-245.
- Takata M et al. (2012). Glucocorticoid-induced TNF receptor-triggered T cells are key modulators for survival/death of neural stem/progenitor cells induced by ischemic stroke. Cell Death Differ 19, 756-767.
- Tivol EA et al. (1995). Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3, 541-547.
- Topalian SL et al. (2012). Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366, 2443-2454.
- Triebel F et al. (1990). LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med 171, 1393-1405.
- van de Weyer PS et al. (2006). A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9. Biochem Biophys Res Commun 351, 571-576.
- van Elsas A et al. (1999). Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med 190, 355-366.
- Wada J and Kanwar YS (1997). Identification and characterization of galectin-9, a novel β-galactoside-binding mammalian lectin. J Biol Chem 272, 6078-6086.
- Waterhouse P et al. (1995). Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270, 985-988.
- Weber JS et al. (2015). Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open label, phase 3 trial. Lancet Oncol 16, 375-384.
- Westin JR et al. (2014). Safety and activity of PD-1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: A single group, open-label, phase 2 trial. Lancet Oncol 15, 69-77.
- Workman CJ and Vignali DA (2003). The CD4-related molecule, LAG-3 (CD223), regulates the expansion of activated T cells. Eur J Immunol 33, 970-979.
- Yu J et al. (2009). Breaking tolerance to self, circulating natural killer cells expressing inhibitory KIR for non-self HLA exhibit effector function after T cell depleted allogeneic hematopoietic cell transplantation. Blood 113, 3875-3884.
- Zhu C et al. (2005). The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol 6, 1245-1252.







