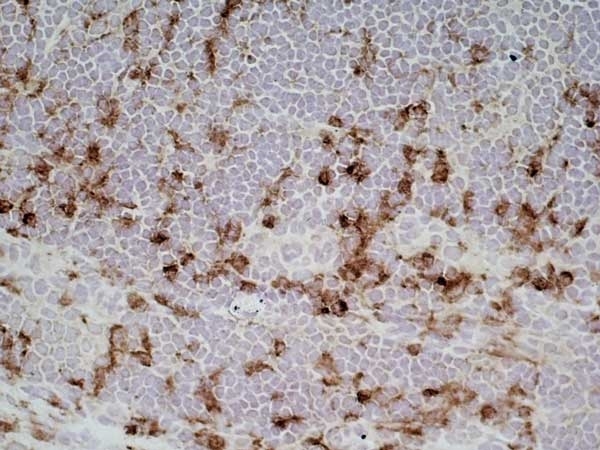
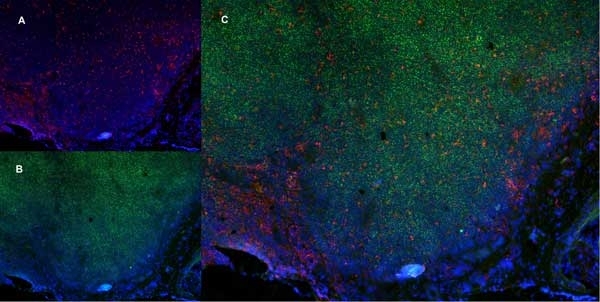
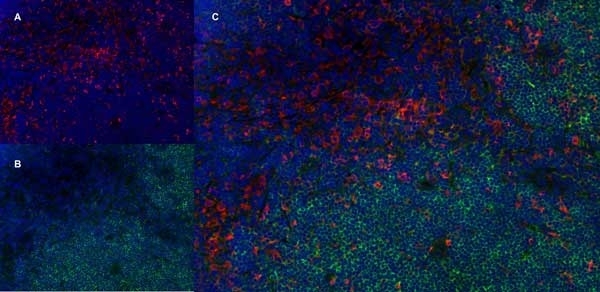
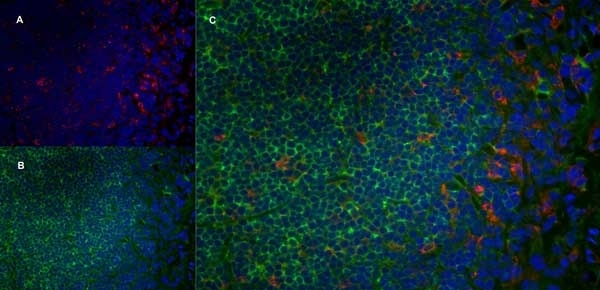
CD68 antibody | ED1




















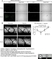


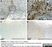




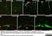

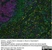
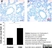
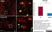
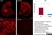
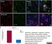

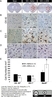










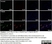

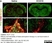

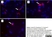
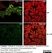
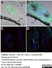








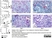


























Mouse anti Rat CD68
- Product Type
- Monoclonal Antibody
- Clone
- ED1
- Isotype
- IgG1
- Specificity
- CD68
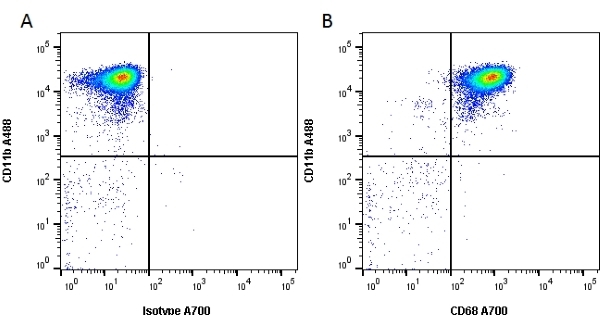
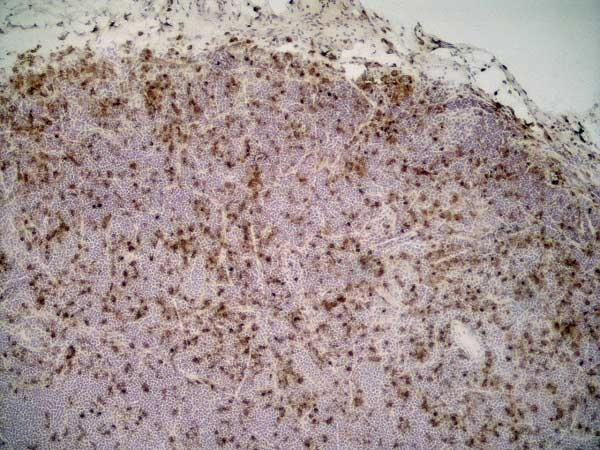
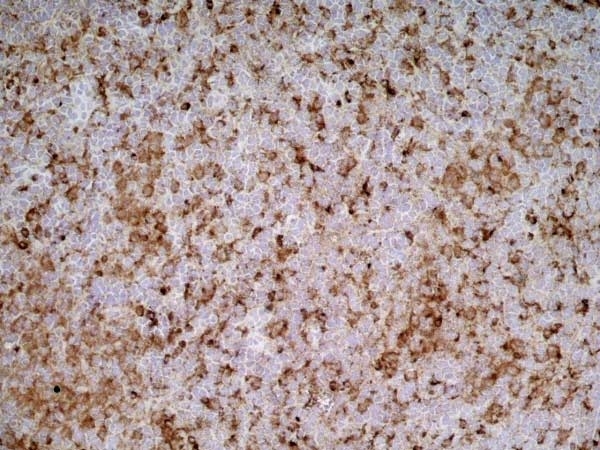
| Mouse anti rat CD68 antibody, clone ED1 recognizes the rat ED1 antigen, a heavily glycosylated protein of ~90 -110 kDa, also known as rat CD68 (Dijkstra et al. 1985). The ED1 antigen is expressed on most macrophages populations, as well as on monocytes and is considered as a pan-macrophage marker in the rat (Damoiseaux et al. 1994). ED1 is expressed predominantly on the lysosomal membrane and lightly on the cell surface (Dijkstra et al. 1985). The expression of ED1 antigen being predominantly cytoplasmic (Dijkstra et al. 1985), flow cytometry results are improved by the use of a membrane permeabilization procedure, such as Leucoperm, prior to staining. |

Our CD68 (ED1) Antibody has been referenced in >580 publications* *Based on June 2020 data from CiteAb's antibody search engine. |
- Target Species
- Rat
- Species Cross-Reactivity
-
Target Species Cross Reactivity Bovine Horse - N.B. Antibody reactivity and working conditions may vary between species.
- Product Form
- Purified IgG - liquid
- Preparation
- MCA341R: Purified IgG prepared by affinity chromatography on Protein A from tissue culture supernatant.
- MCA341GA: Purified IgG prepared by affinity chromatography on Protein A from tissue culture supernatant
- Buffer Solution
- MCA341R: Phosphate buffered saline.
- MCA341GA: Phosphate buffered saline
- Preservative Stabilisers
- MCA341R: 0.09% sodium azide.
-
MCA341GA:
0.09% Sodium Azide - Carrier Free
- Yes
- Immunogen
- Rat spleen cells.
- Approx. Protein Concentrations
- MCA341R: IgG concentration 1.0 mg/ml
- MCA341GA: IgG concentration 0.5 mg/ml
- Fusion Partners
- Spleen cells from immunized BALB/c mice were fused with cells of the SP2/0-Ag14 mouse myeloma cell line.
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Flow Cytometry 1 | 1/50 | 1/100 | |
| Immunofluorescence | |||
| Immunohistology - Frozen | |||
| Immunohistology - Paraffin 2 | 1/100 | ||
| Immunoprecipitation | |||
| Western Blotting |
- 1 Membrane permeabilisation is required for this application.Tthe use of Leucoperm (Product Code BUF09) is recommended for this purpose.
- 2 This product requires protein digestion pre-treatment of paraffin sections e.g. trypsin or pronase
- Flow Cytometry
- Use 10ul of the suggested working dilution to label 1x106 cells in 100ul.
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Mouse IgG1 Negative Control | MCA1209 | F | 0.1 mg |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Mouse IgG1 Negative Control | ||||||
Source Reference
-
Dijkstra, C.D. et al. (1985) The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3.
Immunology. 54 (3): 589-99.
References for CD68 antibody
-
Damoiseaux, J.G. et al. (1994) Rat macrophage lysosomal membrane antigen recognized by monoclonal antibody ED1.
Immunology. 83 (1): 140-7. -
Bauer, J. et al. (1994) Phagocytic activity of macrophages and microglial cells during the course of acute and chronic relapsing experimental autoimmune encephalomyelitis.
J Neurosci Res. 38 (4): 365-75. -
Kornek, B. et al. (2000) Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions.
Am J Pathol. 157: 267-76. -
Bao, F. et al. (2004) Early anti-inflammatory treatment reduces lipid peroxidation and protein nitration after spinal cord injury in rats.
J Neurochem. 88 (6): 1335-44. -
Thom, S.R. et al. (2004) Delayed neuropathology after carbon monoxide poisoning is immune-mediated.
Proc Natl Acad Sci U S A. 101 (37): 13660-5. -
Wu, L. et al. (2004) Dietary approach to attenuate oxidative stress, hypertension, and inflammation in the cardiovascular system.
Proc Natl Acad Sci U S A. 101 (18): 7094-9. -
Pellkofer, H. et al. (2004) Modelling paraneoplastic CNS disease: T-cells specific for the onconeuronal antigen PNMA1 mediate autoimmune encephalomyelitis in the rat.
Brain. 127 (Pt 8): 1822-30. -
Korn, T. et al. (2004) Modulation of effector cell functions in experimental autoimmune encephalomyelitis by leflunomide--mechanisms independent of pyrimidine depletion.
J Leukoc Biol. 76 (5): 950-60.
View The Latest Product References
-
van Dokkum, R.P. et al. (2004) Myocardial infarction enhances progressive renal damage in an experimental model for cardio-renal interaction.
J Am Soc Nephrol. 15 (12): 3103-10. -
Yang, Z.F. et al. (2004) Up-regulation of vascular endothelial growth factor (VEGF) in small-for-size liver grafts enhances macrophage activities through VEGF receptor 2-dependent pathway.
J Immunol. 173 (4): 2507-15. -
Machelska, H. et al. (2004) Selectins and integrins but not platelet-endothelial cell adhesion molecule-1 regulate opioid inhibition of inflammatory pain.
Br J Pharmacol. 142 (4): 772-80. -
Cooperman, S.S. et al. (2005) Microcytic anemia, erythropoietic protoporphyria, and neurodegeneration in mice with targeted deletion of iron-regulatory protein 2.
Blood. 106: 1084-91. -
Deng, X. et al. (2005) Chronic alcohol consumption accelerates fibrosis in response to cerulein-induced pancreatitis in rats.
Am J Pathol. 166: 93-106. -
Cunningham, T.J. et al. (2006) Secreted phospholipase A2 activity in experimental autoimmune encephalomyelitis and multiple sclerosis.
J Neuroinflammation. 3: 26. -
Hawkes, C. et al. (2006) Up-regulation of cation-independent mannose 6-phosphate receptor and endosomal-lysosomal markers in surviving neurons after 192-IgG-saporin administrations into the adult rat brain.
Am J Pathol. 169: 1140-54. -
Wang, H. et al. (2010) Peripheral administration of fetuin-A attenuates early cerebral ischemic injury in rats.
J Cereb Blood Flow Metab. 30: 493-504. -
Munoz-Luque, J. et al. (2007) Regression of fibrosis after chronic stimulation of cannabinoid CB2 receptor in cirrhotic rats.
J Pharmacol Exp Ther. 324: 475-83. -
Candolfi, M. et al. (2007) Intracranial glioblastoma models in preclinical neuro-oncology: neuropathological characterization and tumor progression.
J Neurooncol. 85: 133-48. -
Ryu, J.K. et al. (2008) VEGF receptor antagonist Cyclo-VEGI reduces inflammatory reactivity and vascular leakiness and is neuroprotective against acute excitotoxic striatal insult.
J Neuroinflammation. 5: 18. -
Lohwasser, C. et al. (2009) Role of the receptor for advanced glycation end products in hepatic fibrosis.
World J Gastroenterol. 15: 5789-98. -
Pott Godoy, M.C. et al. (2008) Central and systemic IL-1 exacerbates neurodegeneration and motor symptoms in a model of Parkinson's disease.
Brain. 131: 1880-94. -
Matsuda, K. et al. (2009) Two cases of bovine sarcoma in clinically long-standing lesions.
J Vet Med Sci. 71 (2): 221-4. -
Taylor, S.R. et al. (2009) P2X7 deficiency attenuates renal injury in experimental glomerulonephritis.
J Am Soc Nephrol. 20: 1275-81. -
Patsenker, E. et al. (2009) Pharmacological inhibition of integrin alphavbeta3 aggravates experimental liver fibrosis and suppresses hepatic angiogenesis.
Hepatology. 50: 1501-11. -
Nuki, Y. et al. (2009) Roles of macrophages in flow-induced outward vascular remodeling.
J Cereb Blood Flow Metab. 29: 495-503. -
Zilka, N. et al. (2009) Human misfolded truncated tau protein promotes activation of microglia and leukocyte infiltration in the transgenic rat model of tauopathy.
J. Neuroimmunol. 209: 16-25. -
Jarrett, B.R. et al. (2010) In vivo mapping of vascular inflammation using multimodal imaging.
PLoS One. 5: e13254. -
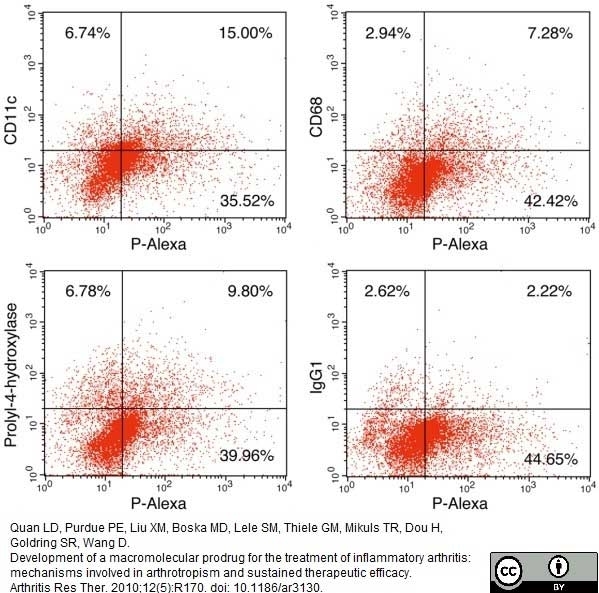
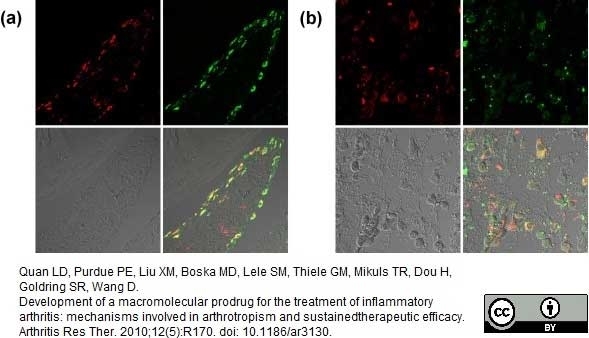
Quan, L.D. et al. (2010) Development of a macromolecular prodrug for the treatment of inflammatory arthritis: mechanisms involved in arthrotropism and sustained therapeutic efficacy.
Arthritis Res Ther.12: R170. -
Halin, S. et al. (2010) Pigment epithelium-derived factor stimulates tumor macrophage recruitment and is downregulated by the prostate tumor microenvironment.
Neoplasia. 12: 336-45. -
Graber, D.J. et al. (2010) Progressive changes in microglia and macrophages in spinal cord and peripheral nerve in the transgenic rat model of amyotrophic lateral sclerosis.
J Neuroinflammation. 7: 8 -
Matsuda, K. et al. (2010) Hemophagocytic histiocytic sarcoma in a Japanese black cow.
Vet Pathol. 47: 339-42. -
Fujita, E. et al. (2010) Statin attenuates experimental anti-glomerular basement membrane glomerulonephritis together with the augmentation of alternatively activated macrophages.
Am J Pathol. 177 (3): 1143-54. -
Bereczky-Veress, B. et al. (2010) Influence of perineurial cells and Toll-like receptors 2 and 9 on Herpes simplex type 1 entry to the central nervous system in rat encephalitis.
PLoS One. 5: e12350. -
Beck, K.D. et al. (2010) Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment.
Brain. 133: 433-47. -
Bedi, A. et al. (2010) Effect of early and delayed mechanical loading on tendon-to-bone healing after anterior cruciate ligament reconstruction.
J Bone Joint Surg Am. 92: 2387-401. -
Hanaoka, M. et al. (2010) Immunomodulatory strategies prevent the development of autoimmune emphysema.
Respir Res. 11:179. -
Marsh, D.R. & Flemming, J.M. (2011) Inhibition of CXCR1 and CXCR2 chemokine receptors attenuates acute inflammation, preserves gray matter and diminishes autonomic dysreflexia after spinal cord injury.
Spinal Cord. 49 (3): 337-44. -
Naito, Y. et al. (2011) Dietary iron restriction prevents hypertensive cardiovascular remodeling in dahl salt-sensitive rats.
Hypertension. 57: 497-504. -
Zhu, W.J. et al. (2011) Intake of water with high levels of dissolved hydrogen (H2) suppresses ischemia-induced cardio-renal injury in Dahl salt-sensitive rats.
Nephrol Dial Transplant. 26: 2112-8. -
Mizuno, T. et al. (2011) Specific collaboration between rat membrane complement regulators Crry and CD59 protects peritoneum from damage by autologous complement activation.
Nephrol Dial Transplant. 26: 1821-30. -
Hamdi, H. et al. (2011) Epicardial adipose stem cell sheets results in greater post-infarction survival than intramyocardial injections.
Cardiovasc Res. 91: 483-91. -
Bousquet, E. et al. (2011) Protective effect of intravitreal administration of tresperimus, an immunosuppressive drug, on experimental autoimmune uveoretinitis.
Invest Ophthalmol Vis Sci. 52: 5414-23. -
Haylor, J.L. et al. (2011) Atorvastatin improving renal ischemia reperfusion injury via direct inhibition of active caspase-3 in rats.
Exp Biol Med (Maywood). 236: 755-63. -
Chen, Y.W. et al. (2011) Dynamic molecular and histopathological changes in the extracellular matrix and inflammation in the transition to heart failure in isolated volume overload
Am J Physiol Heart Circ Physiol. 300: H2251-60. -
Baker, S.C. et al. (2011) Cellular integration and vascularisation promoted by a resorbable, particulate-leached, cross-linked poly(ε-caprolactone) scaffold.
Macromol Biosci. 11 (5): 618-27. -
Liao, T.D. et al. (2011) N-acetyl-seryl-aspartyl-lysyl-proline attenuates renal injury and dysfunction in hypertensive rats with reduced renal mass.
Hypertension. 55: 459-67. -
Paulos, C.M. et al. (2011) Folate-targeted immunotherapy effectively treats established adjuvant and collagen-induced arthritis.
Arthritis Res Ther. 8: R77. -
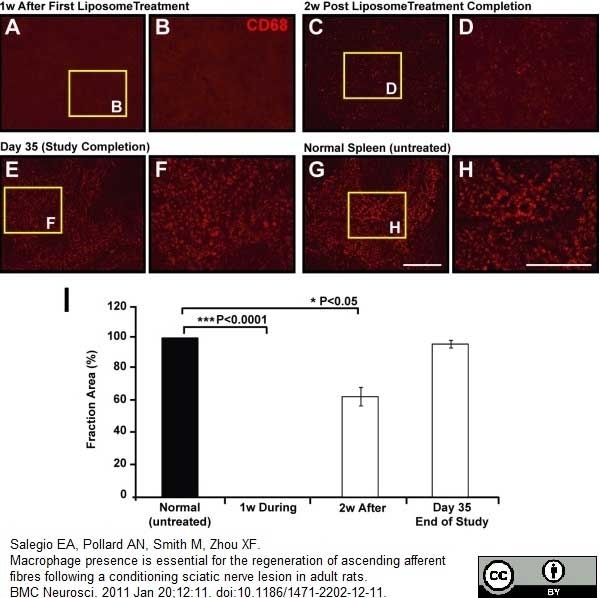
Salegio, E.A. et al. (2011) Macrophage presence is essential for the regeneration of ascending afferent fibres following a conditioning sciatic nerve lesion in adult rats.
BMC Neurosci. 12: 11. -
Teng, B.T. et al. (2011) Protective effect of caspase inhibition on compression-induced muscle damage.
J Physiol. 589: 3349-69. -
McClain, J.A. et al. (2011) Adolescent binge alcohol exposure induces long-lasting partial activation of microglia.
Brain Behav Immun. 25 Suppl 1: S120-8. -
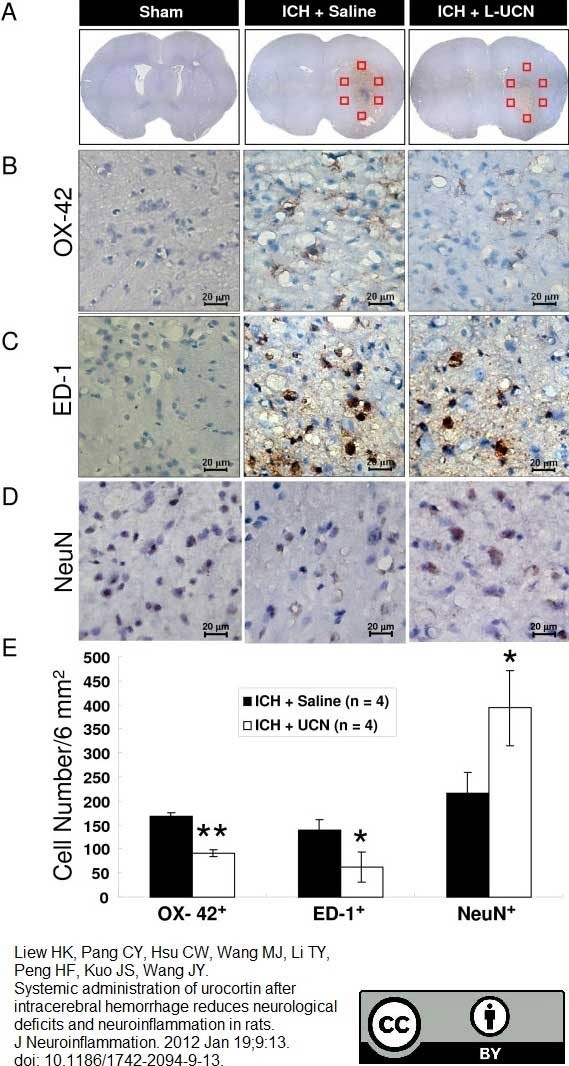
Liew, H.K. et al. (2012) Systemic administration of urocortin after intracerebral hemorrhage reduces neurological deficits and neuroinflammation in rats.
J Neuroinflammation. 9: 13. -
Glorie, L.L. et al. (2012) DPP4 inhibition improves functional outcome after renal ischemia-reperfusion injury.
Am J Physiol Renal Physiol. 303: F681-8. -
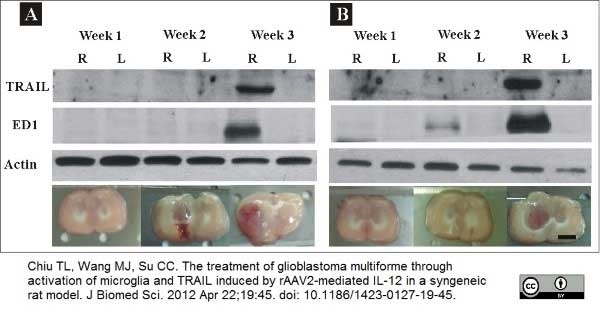
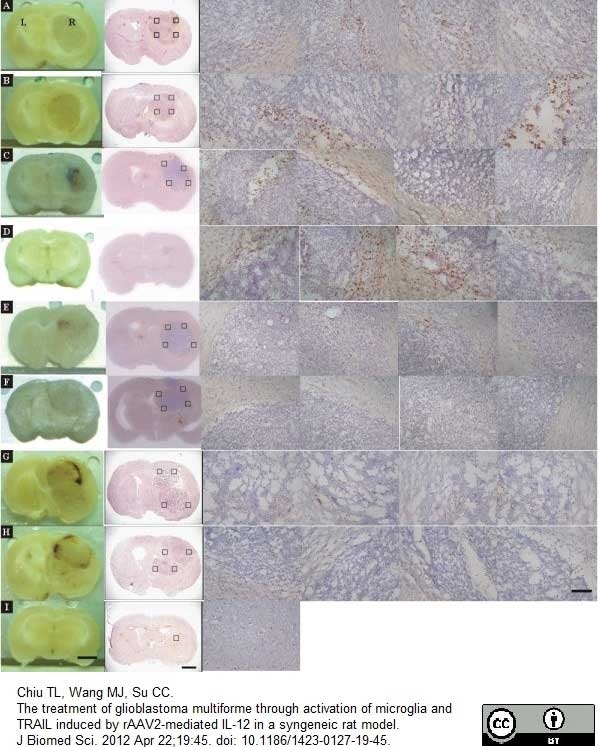
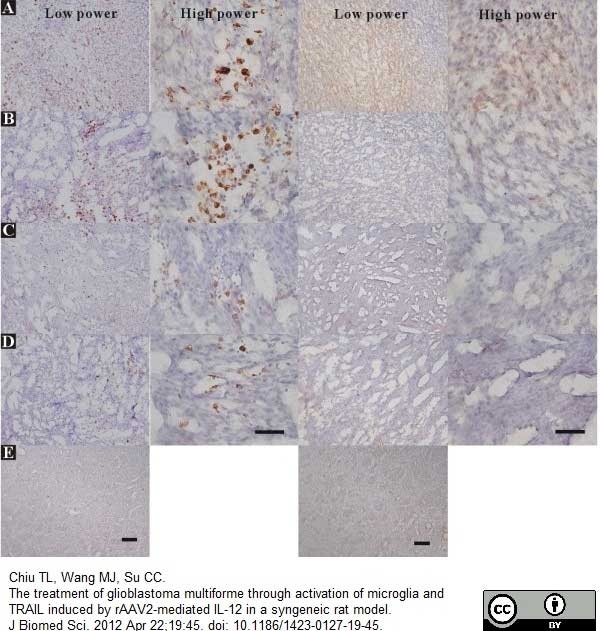
Chiu, T.L. et al. (2012) The treatment of glioblastoma multiforme through activation of microglia and TRAIL induced by rAAV2-mediated IL-12 in a syngeneic rat model.
J Biomed Sci. 19: 45. -
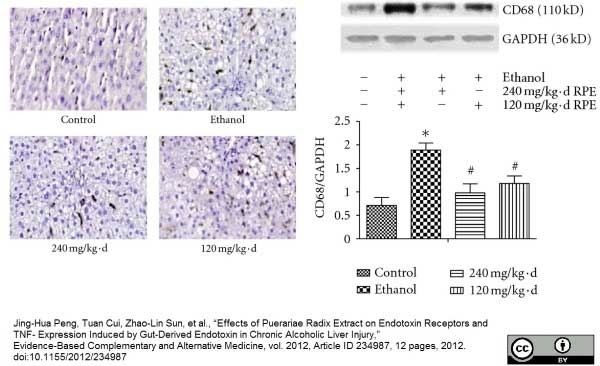
Peng, J.H. et al. (2012) Effects of Puerariae Radix Extract on Endotoxin Receptors and TNF-α Expression Induced by Gut-Derived Endotoxin in Chronic Alcoholic Liver Injury.
Evid Based Complement Alternat Med. 2012: 234987. -
Tian, Y.F. et al. (2013) Lipoic acid suppresses portal endotoxemia-induced steatohepatitis and pancreatic inflammation in rats.
World J Gastroenterol. 19 (18): 2761-71. -
Xiang, Y. et al. (2013) L-carnitine protects against cyclosporine-induced pancreatic and renal injury in rats.
Transplant Proc. 45 (8): 3127-34. -
Wang-Rosenke, Y. et al. (2013) Tyrosine kinases inhibition by Imatinib slows progression in chronic anti-thy1 glomerulosclerosis of the rat.
BMC Nephrol. 14: 223. -
Dort, J. et al. (2013) Beneficial Effects of Cod Protein on Inflammatory Cell Accumulation in Rat Skeletal Muscle after Injury Are Driven by Its High Levels of Arginine, Glycine, Taurine and Lysine.
PLoS One. 8: e77274. -
Chang, C.Y. et al. (2013) Docosahexaenoic acid reduces cellular inflammatory response following permanent focal cerebral ischemia in rats.
J Nutr Biochem. 24 (12): 2127-37. -
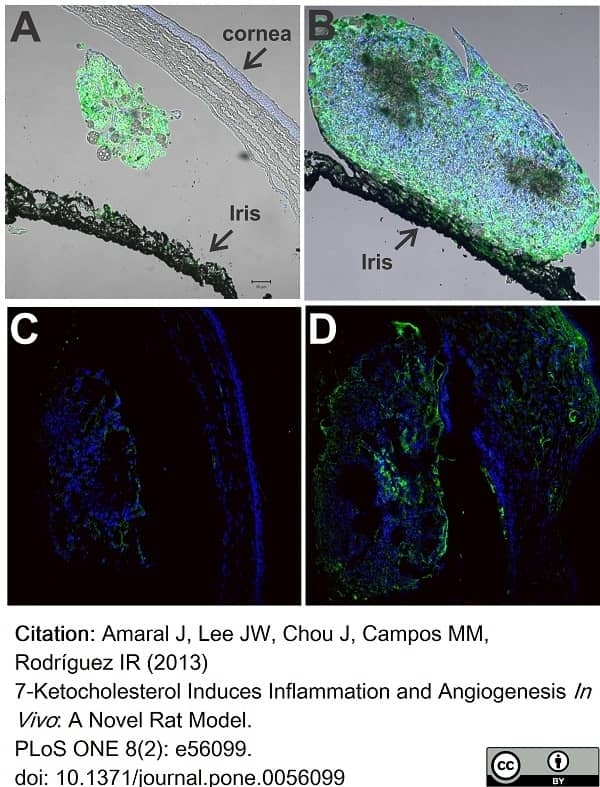
Amaral, J. et al. (2013) 7-Ketocholesterol induces inflammation and angiogenesis in vivo: a novel rat model.
PLoS One. 8 (2): e56099. -
Layachi, S. et al. (2013) Role of cellular effectors in the emergence of ventilation defects during allergic bronchoconstriction.
J Appl Physiol (1985). 115 (7): 1057-64. -
Huang, H.L. et al. (2013) Krüppel-like factor 5 associates with melamine-cyanurate crystal-induced nephritis in rats.
Nephrol Dial Transplant. 28 (10): 2477-83. -
Wei, X. et al. (2014) Dural fibroblasts play a potential role in headache pathophysiology.
Pain. 155: 1238-44. -
Hiraoka, M. et al. (2014) Increase of lysosomal phospholipase A2 in aqueous humor by uveitis.
Exp Eye Res. 118: 13-9. -
Kim, Y.H. et al. (2014) Enhancement of bone regeneration by dual release of a macrophage recruitment agent and platelet-rich plasma from gelatin hydrogels.
Biomaterials. 35 (1): 214-24. -
Xu, X. et al. (2014) Aging aggravates long-term renal ischemia-reperfusion injury in a rat model.
J Surg Res. 187 (1): 289-96. -
Sakuraya, K. et al. (2014) The synergistic effect of mizoribine and a direct renin inhibitor, aliskiren, on unilateral ureteral obstruction induced renal fibrosis in rats.
J Urol. 191 (4): 1139-46. -
Arnold, C.E. et al. (2014) A critical role for suppressor of cytokine signalling 3 in promoting M1 macrophage activation and function in vitro and in vivo.
Immunology. 141 (1): 96-110. -
DeRamon, L. et al. (2015) CD154-CD40 T-cell co-stimulation pathway is a key mechanism in kidney ischemia-reperfusion injury.
Kidney Int. 88 (3): 538-49. -
Chang, C.Y. et al. (2015) Tetramethylpyrazine inhibits neutrophil activation following permanent cerebral ischemia in rats.
Biochem Biophys Res Commun. 463 (3): 421-7. -
Carrillo-de Sauvage, M.A. et al. (2015) The neuroprotective agent CNTF decreases neuronal metabolites in the rat striatum: an in vivo multimodal magnetic resonance imaging study.
J Cereb Blood Flow Metab. 35 (6): 917-21. -
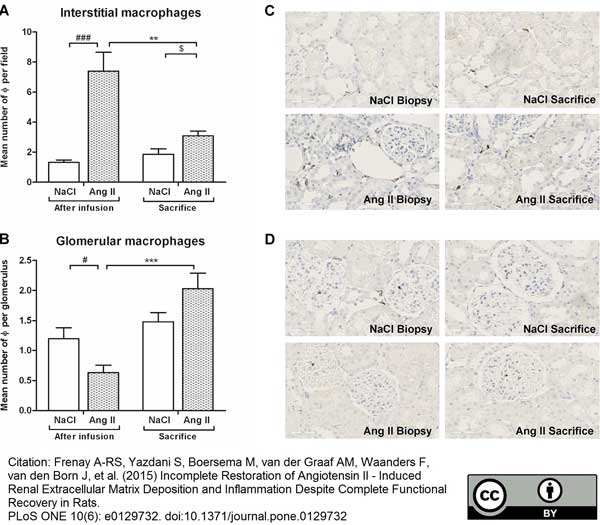
Menzies, R.I. et al. (2015) Inhibition of the purinergic P2X7 receptor improves renal perfusion in angiotensin-II-infused rats.
Kidney Int. 88 (5): 1079-87. -
Paulsen, I.M. et al. (2015) A single simple procedure for dewaxing, hydration and heat-induced epitope retrieval (HIER) for immunohistochemistry in formalin fixed paraffin-embedded tissue.
Eur J Histochem. 59 (4): 2532. -
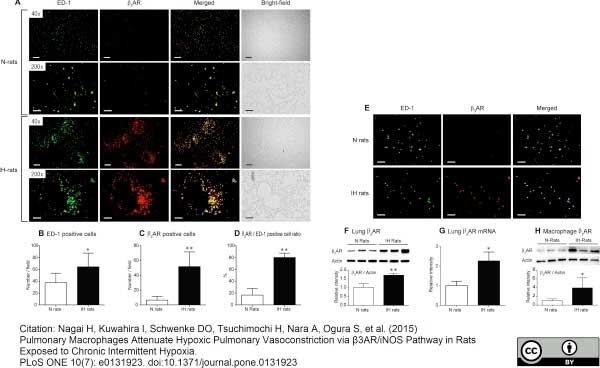
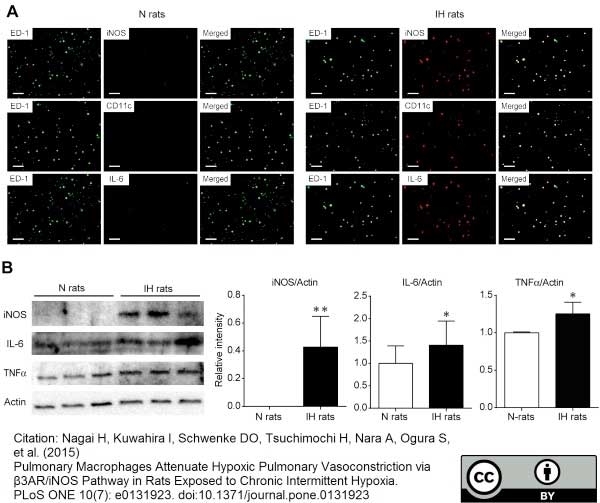
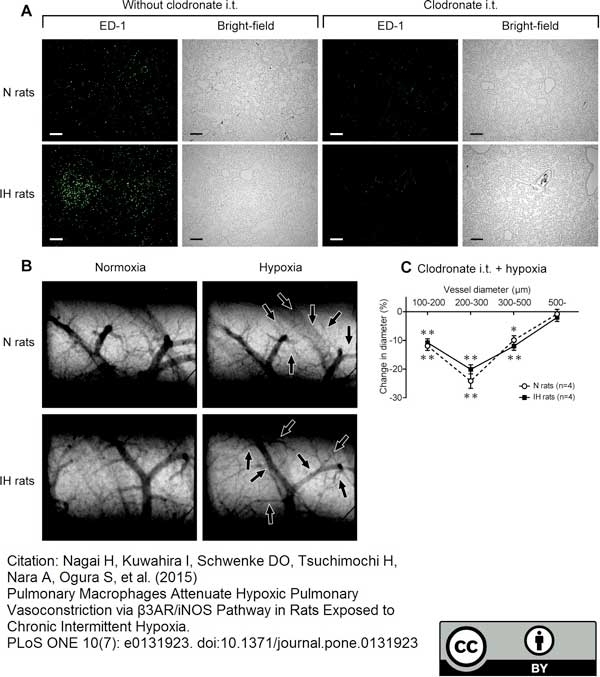
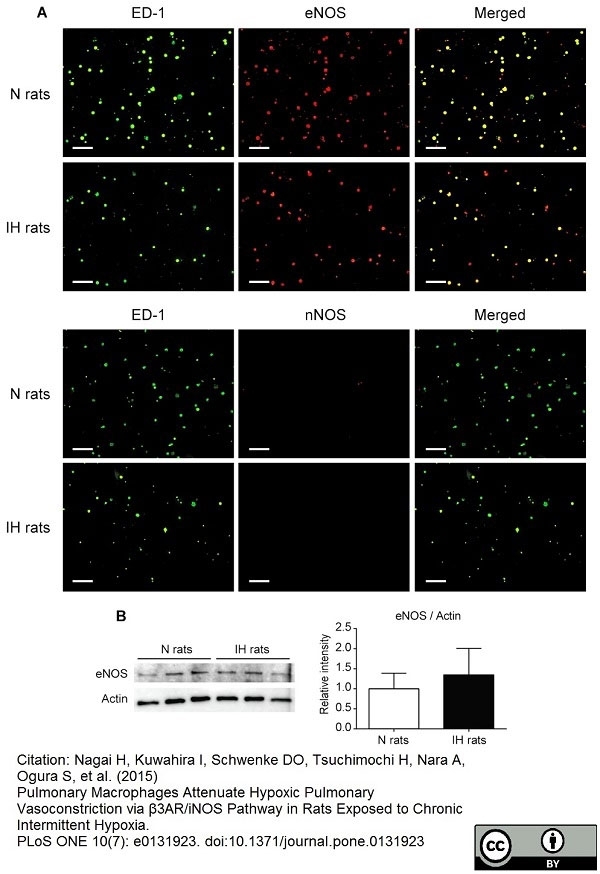
Nagai, H. et al. (2015) Pulmonary Macrophages Attenuate Hypoxic Pulmonary Vasoconstriction via β3AR/iNOS Pathway in Rats Exposed to Chronic Intermittent Hypoxia.
PLoS One. 10 (7): e0131923. -
Han, T.T. et al. (2015) Adipose-derived stromal cells mediate in vivo adipogenesis, angiogenesis and inflammation in decellularized adipose tissue bioscaffolds.
Biomaterials. 72: 125-37. -
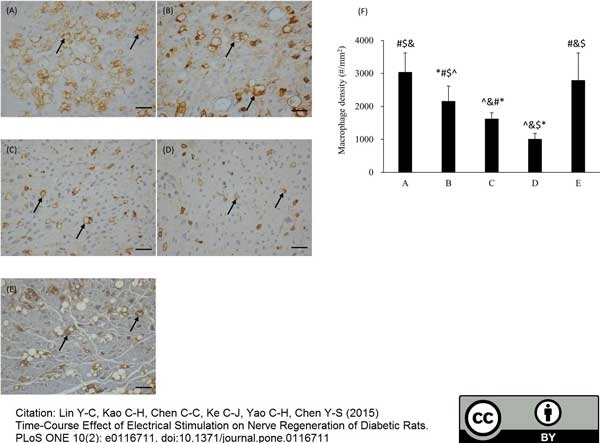
Lin, Y.C. et al. (2015) Time-course effect of electrical stimulation on nerve regeneration of diabetic rats.
PLoS One. 10: e0116711. -
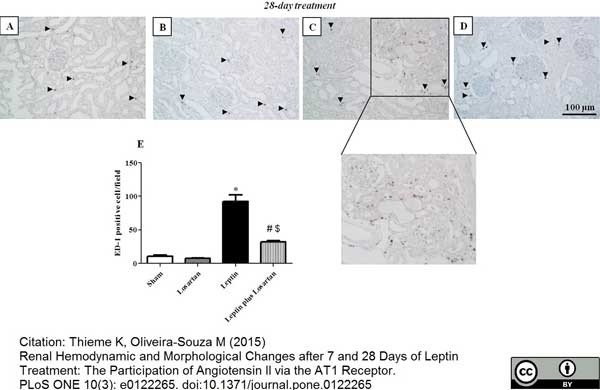
Thieme, K. & Oliveira-Souza, M. (2015) Renal Hemodynamic and Morphological Changes after 7 and 28 Days of Leptin Treatment: The Participation of Angiotensin II via the AT1 Receptor.
PLoS One. 10 (3): e0122265. -
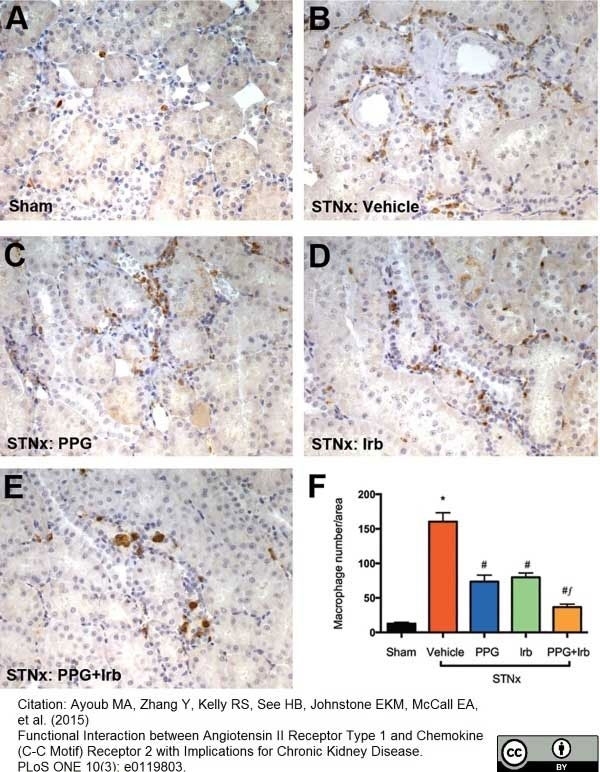
Ayoub, M.A. et al. (2015) Functional Interaction between Angiotensin II Receptor Type 1 and Chemokine (C-C Motif) Receptor 2 with Implications for Chronic Kidney Disease.
PLoS One. 10 (3): e0119803. -
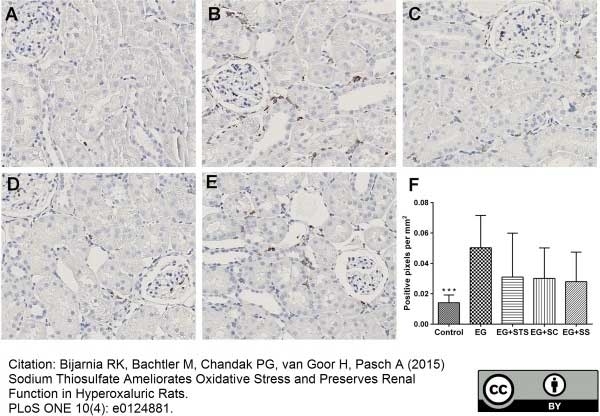
Bijarnia, R.K. et al. (2015) Sodium thiosulfate ameliorates oxidative stress and preserves renal function in hyperoxaluric rats.
PLoS One. 10 (4): e0124881. -
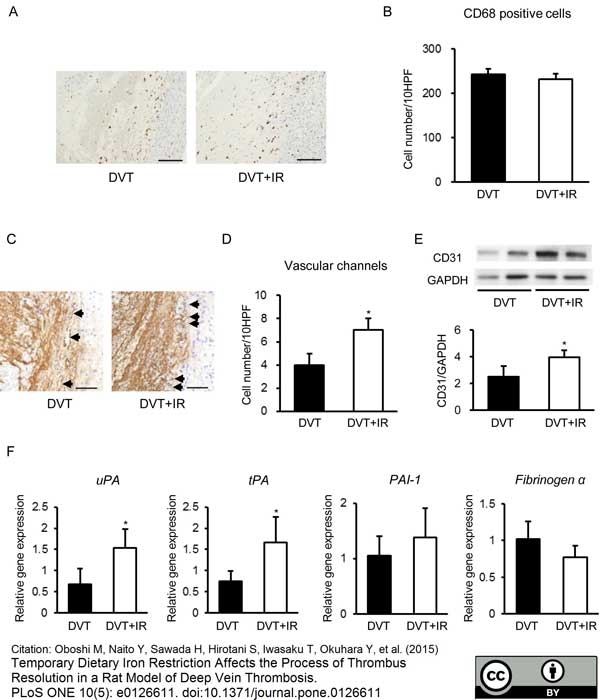
Oboshi, M. et al. (2015) Temporary dietary iron restriction affects the process of thrombus resolution in a rat model of deep vein thrombosis.
PLoS One. 10 (5): e0126611. -
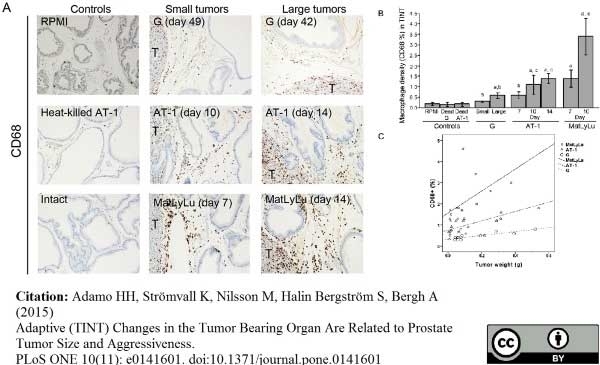
Adamo, H.H. et al. (2015) Adaptive (TINT) Changes in the Tumor Bearing Organ Are Related to Prostate Tumor Size and Aggressiveness.
PLoS One. 10 (11): e0141601. -
Cha, S.J. et al. (2016) Identification of GAPDH on the surface of Plasmodium sporozoites as a new candidate for targeting malaria liver invasion.
J Exp Med. 213 (10): 2099-112. -
Murata, M. et al. (2016) Surfactant protein D is a useful biomarker for monitoring acute lung injury in rats.
Exp Lung Res. 42 (6): 314-21. -
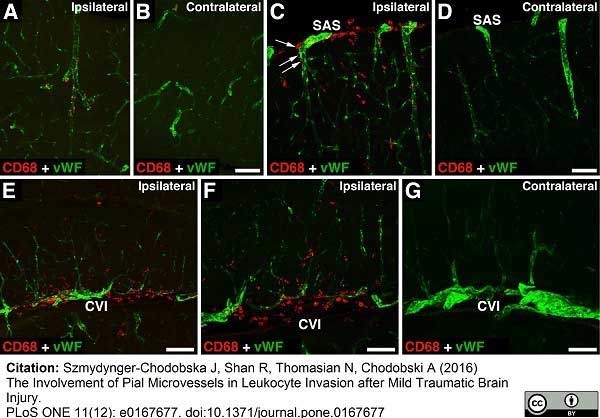
Szmydynger-Chodobska, J. et al. (2016) The Involvement of Pial Microvessels in Leukocyte Invasion after Mild Traumatic Brain Injury.
PLoS One. 11 (12): e0167677. -
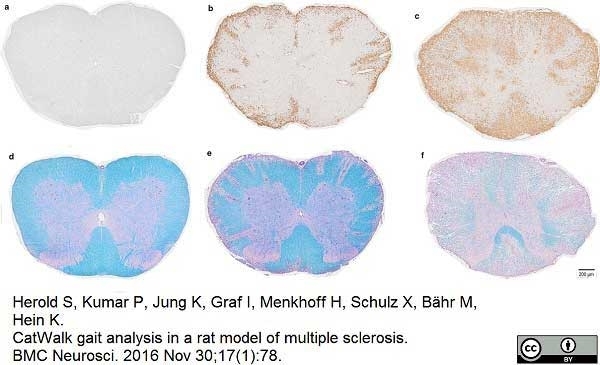
Herold, S. et al. (2016) CatWalk gait analysis in a rat model of multiple sclerosis.
BMC Neurosci. 17 (1): 78. -
Cóndor JM et al. (2016) Treatment With Human Wharton's Jelly-Derived Mesenchymal Stem Cells Attenuates Sepsis-Induced Kidney Injury, Liver Injury, and Endothelial Dysfunction.
Stem Cells Transl Med. 5 (8): 1048-57. -
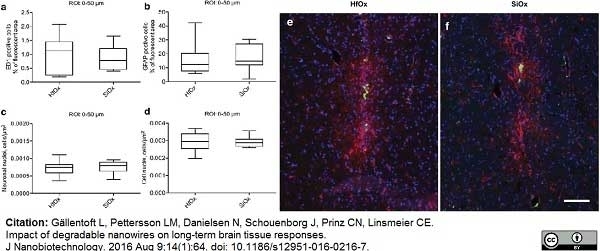
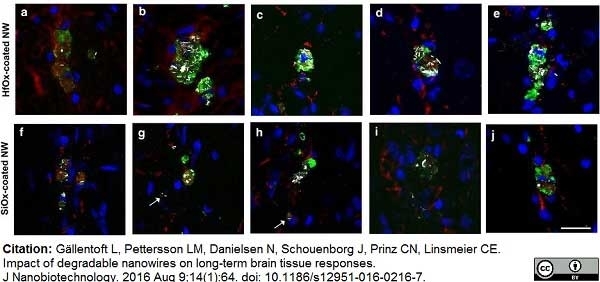
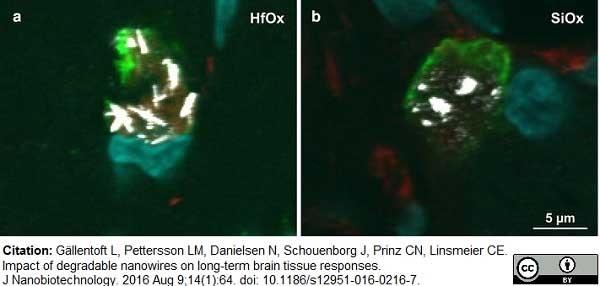
Gällentoft, L. et al. (2016) Impact of degradable nanowires on long-term brain tissue responses.
J Nanobiotechnology. 14 (1): 64. -
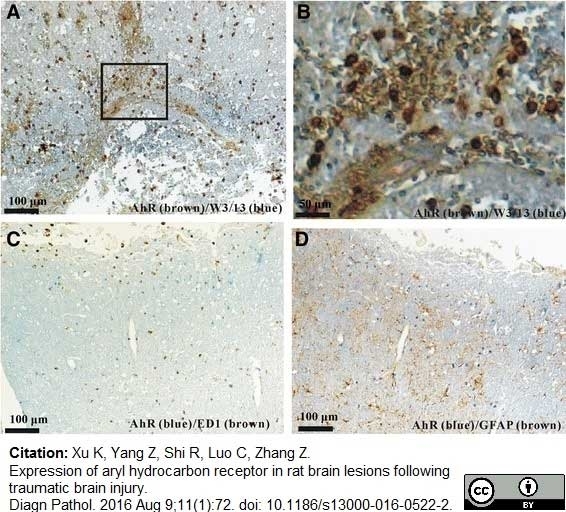
Xu K et al. (2016) Expression of aryl hydrocarbon receptor in rat brain lesions following traumatic brain injury.
Diagn Pathol. 11 (1): 72. -
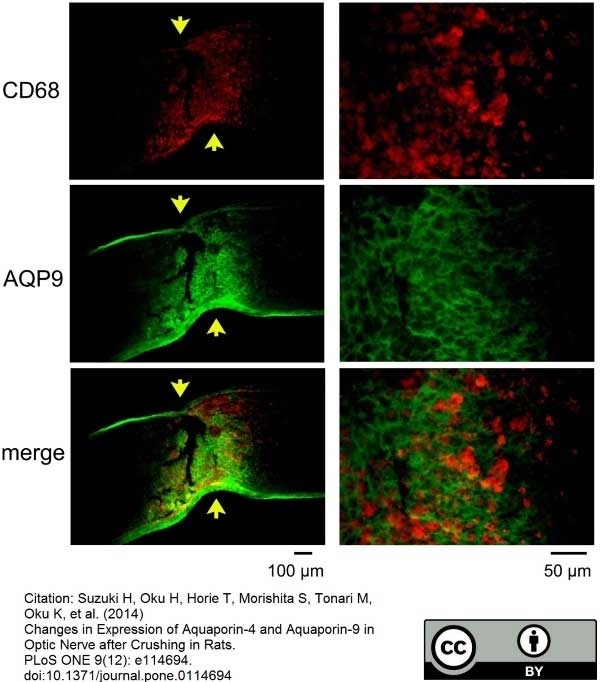
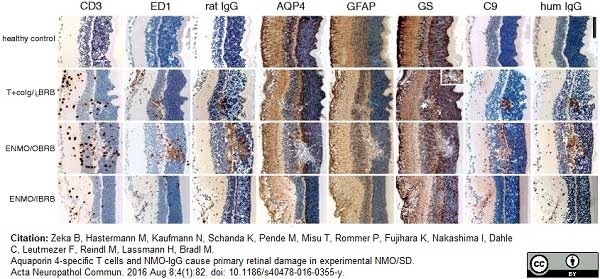
Zeka, B. et al. (2016) Aquaporin 4-specific T cells and NMO-IgG cause primary retinal damage in experimental NMO/SD.
Acta Neuropathol Commun. 4 (1): 82. -
Hashmat, S. et al. (2016) Interleukin-6 inhibition attenuates hypertension and associated renal damage in Dahl salt-sensitive rats.
Am J Physiol Renal Physiol. 311 (3): F555-61. -
Ibarra, V. et al. (2016) This paper is a winner in the Undergraduate category for the SFB awards: Evaluation of the tissue response to alginate encapsulated islets in an omentum pouch model.
J Biomed Mater Res A. 104 (7): 1581-90. -
Kühne, L. et al. (2017) Renal allograft rejection, lymphocyte infiltration, and de novo donor-specific antibodies in a novel model of non-adherence to immunosuppressive therapy.
BMC Immunol. 18 (1): 52. -
Fontana, J. et al. (2017) Impact of Steroids on the Inflammatory Response after Ischemic Acute Kidney Injury in Rats.
Indian J Nephrol. 27 (5): 365-71. -
Kanamori, H. et al. (2017) Influence of nicotine on choline-deficient, L-amino acid-defined diet-induced non-alcoholic steatohepatitis in rats.
PLoS One. 12 (6): e0180475. -
Wang, M. et al. (2017) Characterization of the Micro-Environment of the Testis that Shapes the Phenotype and Function of Testicular Macrophages.
J Immunol. 198 (11): 4327-40. -
Xue, Y. et al. (2017) Hydroxyapatite nanoparticle-induced mitochondrial energy metabolism impairment in liver cells: in vitro and in vivo studies.
J Appl Toxicol. 37 (8): 1004-1016. -
Londono, R. et al. (2017) The effect of cell debris within biologic scaffolds upon the macrophage response.
J Biomed Mater Res A. 105 (8): 2109-18. -
Landeck, N. et al. (2017) Toxic effects of human and rodent variants of alpha-synuclein in vivo.
Eur J Neurosci. 45 (4): 536-47. -
Haba, D. et al. (2017) Morphological study on the pressure ulcer-like dermal lesions formed in the rat heel skin after transection of the sciatic nerves.
Acta Histochem. 119 (1): 39-47. -
Aarts, S.A.B.M. et al. (2017) Inhibition of CD40-TRAF6 interactions by the small molecule inhibitor 6877002 reduces neuroinflammation.
J Neuroinflammation. 14 (1): 105. -
Faleiros, C.M. et al. (2017) Effects of previous physical training on adriamycin nephropathy and its relationship with endothelial lesions and angiogenesis in the renal cortex.
Life Sci. 169: 43-51. -
Russo, E.R. et al. (2018) Oral administration of powdered dried rhizomes of Curcuma longa. L. (turmeric, Zingiberaceae.) is effective in the treatment of doxorubicin-induced kidney injury in rats.
Phytother Res. 32 (12): 2408-16. -
Kodam, A. et al. (2019) A role for astrocyte-derived amyloid β peptides in the degeneration of neurons in an animal model of temporal lobe epilepsy.
Brain Pathol. 29 (1): 28-44. -
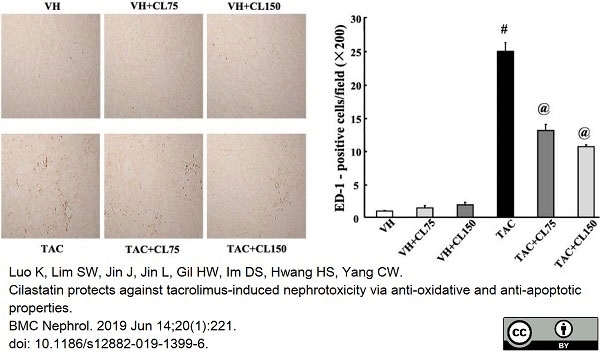
Luo, K. et al. (2019) Cilastatin protects against tacrolimus-induced nephrotoxicity via anti-oxidative and anti-apoptotic properties.
BMC Nephrol. 20 (1): 221. -
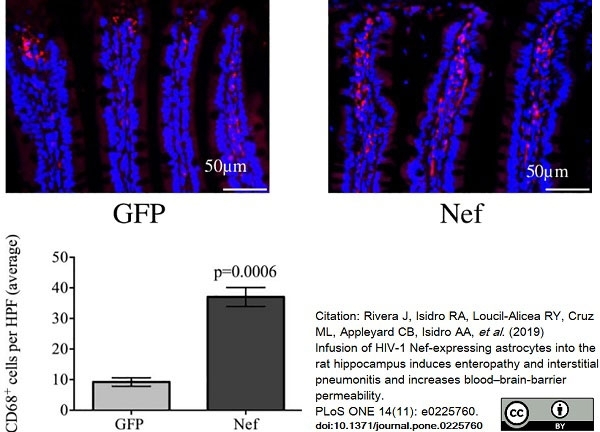
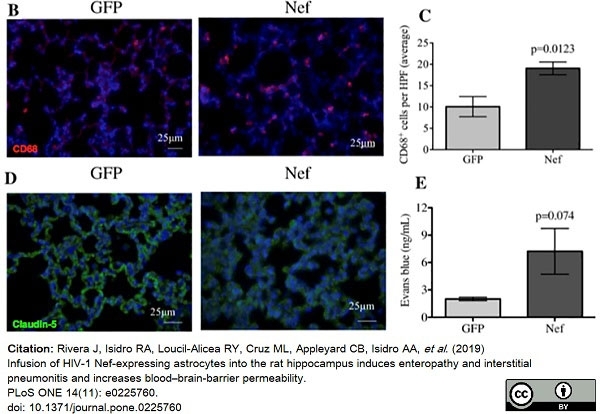
Rivera, J. et al. (2019) Infusion of HIV-1 Nef-expressing astrocytes into the rat hippocampus induces enteropathy and interstitial pneumonitis and increases blood-brain-barrier permeability.
PLoS One. 14 (11): e0225760. -
Ornellas, F.M. et al. (2019) Mesenchymal Stromal Cells Induce Podocyte Protection in the Puromycin Injury Model.
Sci Rep. 9 (1): 19604. -
Grad, E. et al. (2019) Monocyte Modulation by Liposomal Alendronate Improves Cardiac Healing in a Rat Model of Myocardial Infarction
Regen Eng Transl Med. 5 (3): 280-9. -
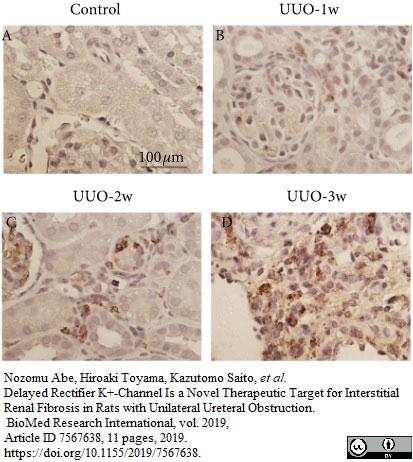
Abe, N. et al. (2019) Delayed Rectifier K+-Channel Is a Novel Therapeutic Target for Interstitial Renal Fibrosis in Rats with Unilateral Ureteral Obstruction.
Biomed Res Int. 2019: 7567638. -
Silva, F.M.O. et al. (2019) Tamoxifen and bone morphogenic protein-7 modulate fibrosis and inflammation in the peritoneal fibrosis model developed in uremic rats.
Mol Med. 25 (1): 41. -
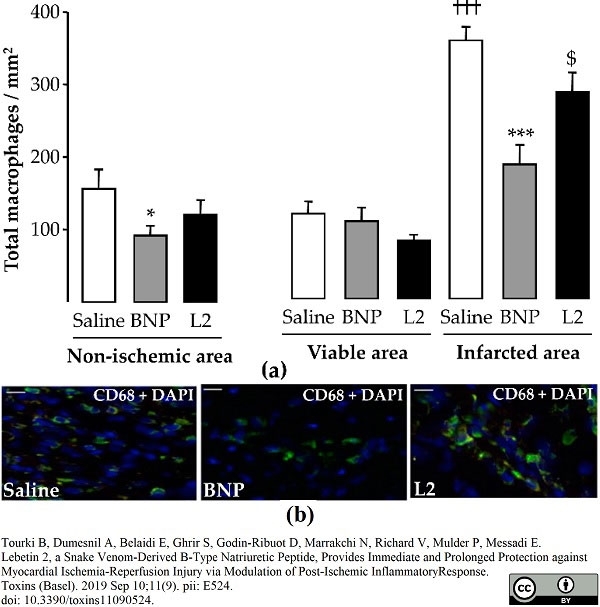
Tourki, B. et al. (2019) Lebetin 2, a Snake Venom-Derived B-Type Natriuretic Peptide, Provides Immediate and Prolonged Protection against Myocardial Ischemia-Reperfusion Injury via Modulation of Post-Ischemic Inflammatory Response.
Toxins (Basel). 11(9):524. -
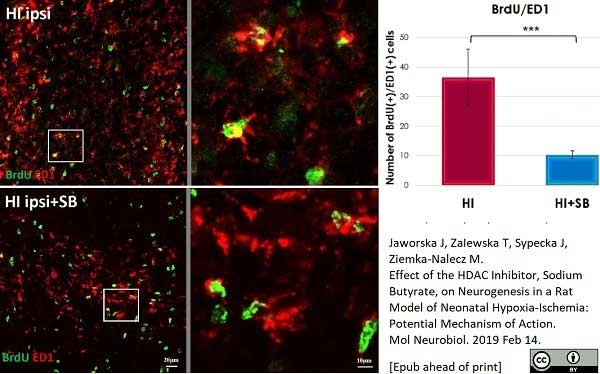
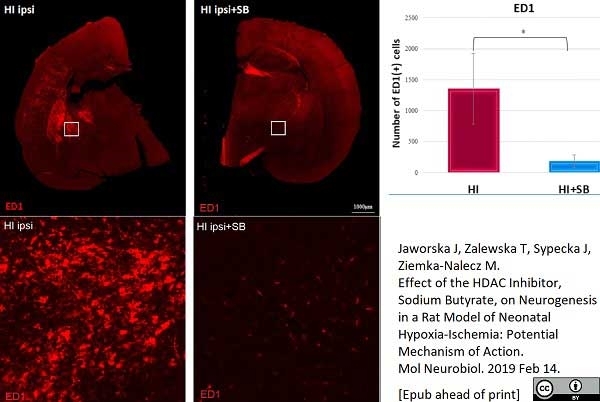
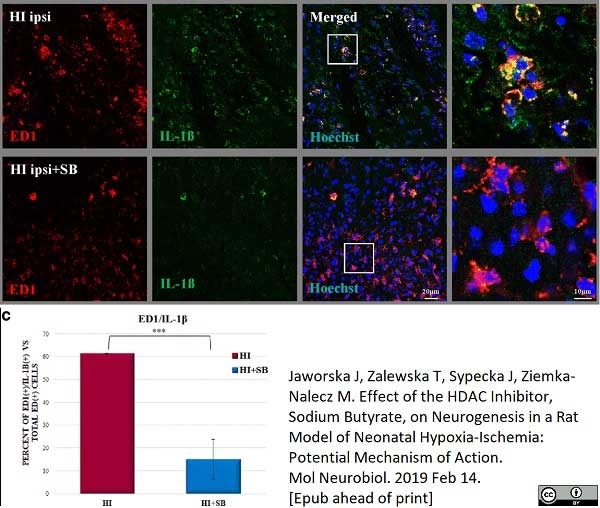
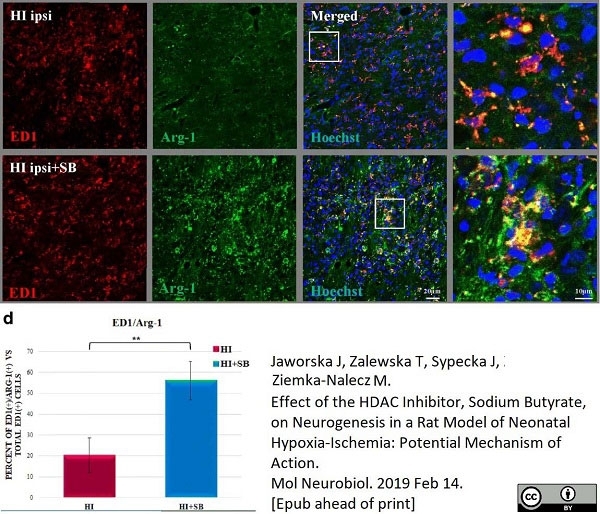
Jaworska, J. et al. (2019) Effect of the HDAC Inhibitor, Sodium Butyrate, on Neurogenesis in a Rat Model of Neonatal Hypoxia-Ischemia: Potential Mechanism of Action.
Mol Neurobiol. 56 (9): 6341-70. -
Hoff, U. et al. (2019) A synthetic epoxyeicosatrienoic acid analogue prevents the initiation of ischemic acute kidney injury.
Acta Physiol (Oxf). 227 (2): e13297. -
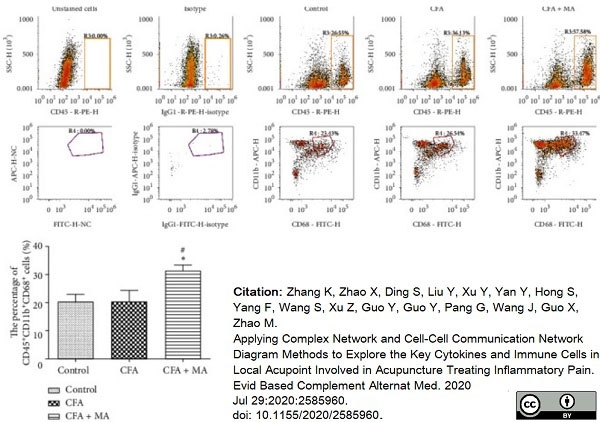
Zhang, K. et al. (2020) Applying Complex Network and Cell-Cell Communication Network Diagram Methods to Explore the Key Cytokines and Immune Cells in Local Acupoint Involved in Acupuncture Treating Inflammatory Pain.
Evid Based Complement Alternat Med. 2020: 2585960. -
Tanaka, J. et al. (2020) Generation of CSF1-Independent Ramified Microglia-Like Cells from Leptomeninges In Vitro..
Cells. 10 (1): 24. -
Haase, N. et al. (2020) RNA interference therapeutics targeting angiotensinogen ameliorate preeclamptic phenotype in rodent models.
J Clin Invest. 130 (6): 2928-42. -
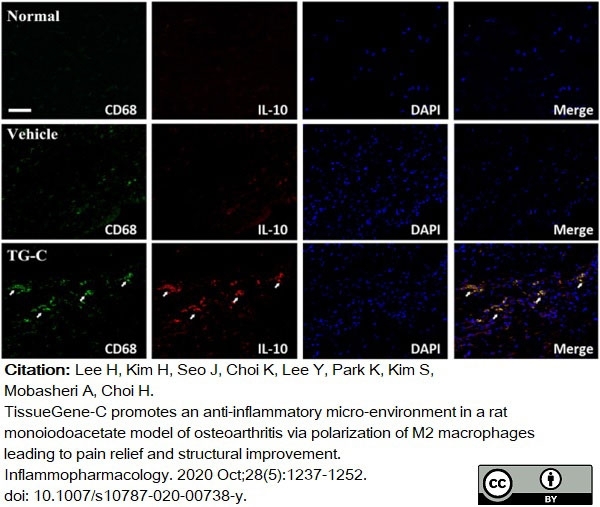
Lee, H. et al. (2020) TissueGene-C promotes an anti-inflammatory micro-environment in a rat monoiodoacetate model of osteoarthritis via polarization of M2 macrophages leading to pain relief and structural improvement.
Inflammopharmacology. 28 (5): 1237-52. -
Yao, X. et al. (2021) Acellular Collagen Scaffold With Basic Fibroblast Growth Factor for Repair of Traumatic Tympanic Membrane Perforation in a Rat Model.
Otolaryngol Head Neck Surg. 164 (2): 381-90. -
Li, R. et al. (2020) Bone marrow mesenchymal stem cell-derived exosomal microRNA-124-3p attenuates neurological damage in spinal cord ischemia-reperfusion injury by downregulating Ern1 and promoting M2 macrophage polarization.
Arthritis Res Ther. 22 (1): 75. -
Choi, Y. et al. (2020) Immunohistochemical analysis of periostin in the hearts of Lewis rats with experimental autoimmune myocarditis.
J Vet Med Sci. 82 (10): 1545-50. -
Arai, M. et al. (2020) Morphological and phenotypical diversity of eosinophils in the rat ileum.
Cell Tissue Res. 381 (3): 439-450. -
Torigoe, K. et al. (2020) Hexapeptide derived from prothymosin alpha attenuates cisplatin-induced acute kidney injury.
Clin Exp Nephrol. 24 (5): 411-9. -
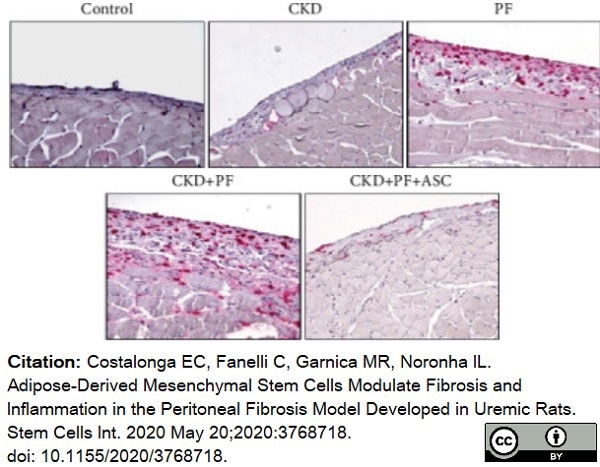
Costalonga, E.C. et al. (2020) Adipose-Derived Mesenchymal Stem Cells Modulate Fibrosis and Inflammation in the Peritoneal Fibrosis Model Developed in Uremic Rats.
Stem Cells Int. 2020: 3768718. -
Cohrs, G. et al. (2020) Expression Patterns of Hypoxia-Inducible Factors, Proinflammatory, and Neuroprotective Cytokines in Neuroepithelial Tissues of Lumbar Spinal Lipomas-A Pilot Study.
World Neurosurg. 141: e633-e644. -
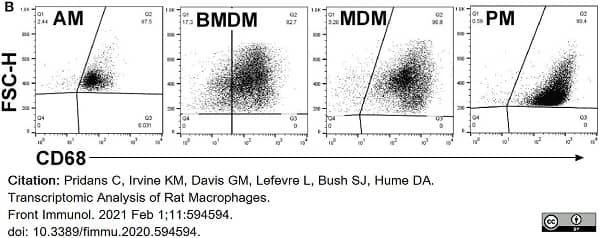
Pridans, C. et al. (2020) Transcriptomic Analysis of Rat Macrophages.
Front Immunol. 11: 594594. -
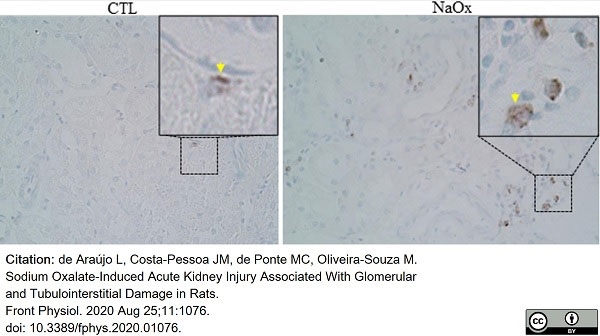
de Araújo L.A. et al. (2020) Sodium Oxalate-Induced Acute Kidney Injury Associated With Glomerular and Tubulointerstitial Damage in Rats.
Front Physiol. 11: 1076. -
Jahandideh, A. et al. (2020) Folate Receptor β-Targeted PET Imaging of Macrophages in Autoimmune Myocarditis.
J Nucl Med. 61 (11): 1643-9. -
Zhang, L.Y. et al. (2020) Microglia exacerbate white matter injury via complement C3/C3aR pathway after hypoperfusion.
Theranostics. 10 (1): 74-90. -
Kaur, G. et al. (2020) Neonatal Pig Sertoli Cells Survive Xenotransplantation by Creating an Immune Modulatory Environment Involving CD4 and CD8 Regulatory T Cells.
Cell Transplant. 29: 963689720947102. -
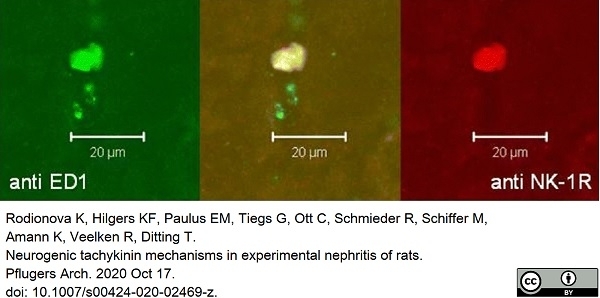
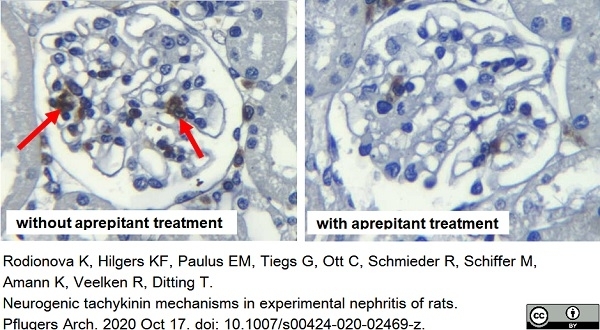
Rodionova, K. et al. (2020) Neurogenic tachykinin mechanisms in experimental nephritis of rats.
Pflugers Arch. 472 (12): 1705-17. -
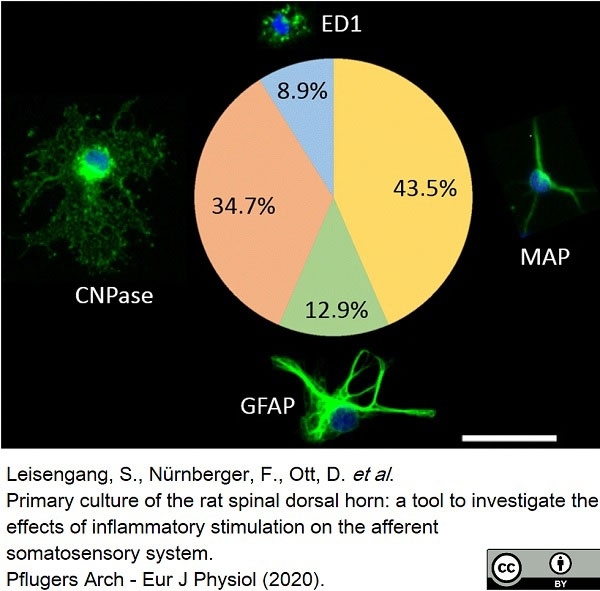
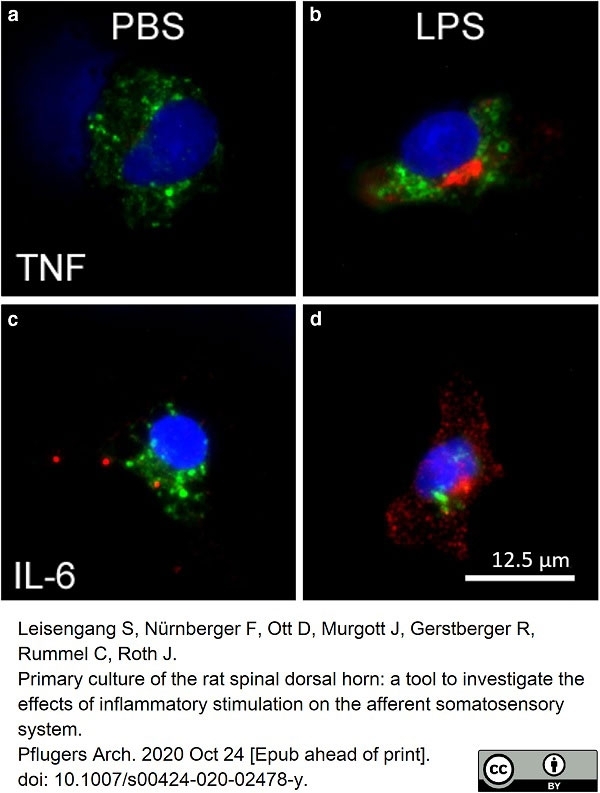
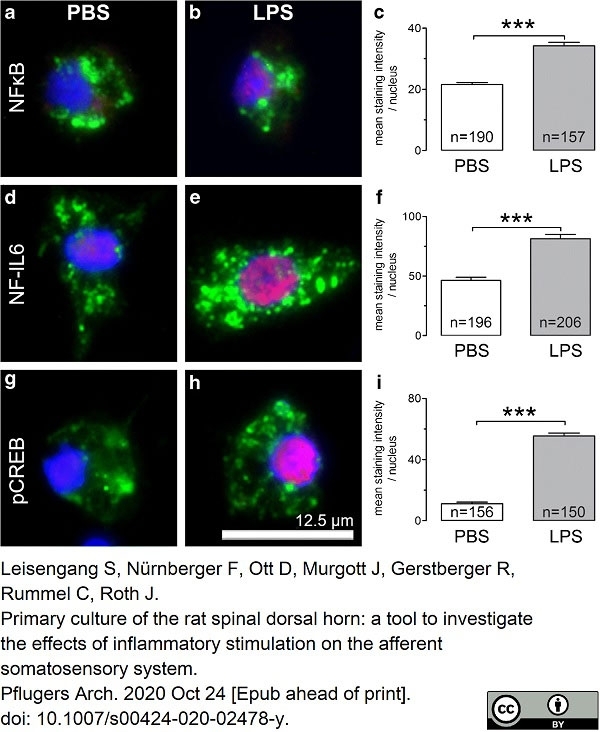
Leisengang, S. et al. (2020) Primary culture of the rat spinal dorsal horn: a tool to investigate the effects of inflammatory stimulation on the afferent somatosensory system.
Pflugers Arch. 472 (12): 1769-82. -
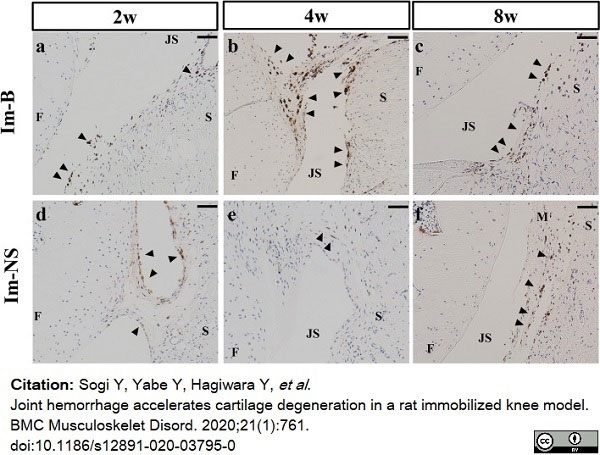
Sogi, Y. et al. (2020) Joint hemorrhage accelerates cartilage degeneration in a rat immobilized knee model.
BMC Musculoskelet Disord. 21 (1): 761. -
Muri, L. et al.. (2020) Repetitive transcranial magnetic stimulation activates glial cells and inhibits neurogenesis after pneumococcal meningitis.
PLoS ONE 15(9): e0232863. -
Zhang, Z. et al. (2020) Mesenchymal Stem Cells Promote the Resolution of Cardiac Inflammation After Ischemia Reperfusion Via Enhancing Efferocytosis of Neutrophils.
J Am Heart Assoc. 9 (5): e014397. -
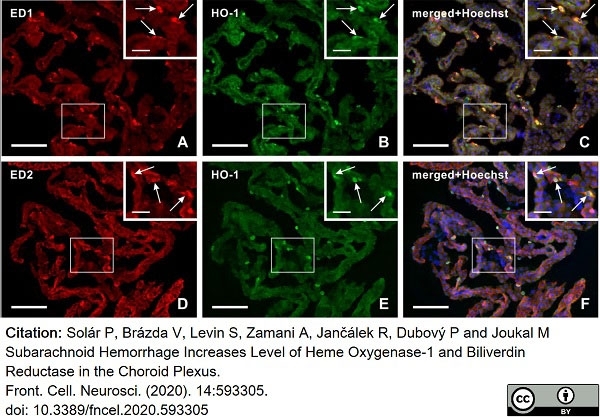
Solár, P. et al. (2020) Subarachnoid Hemorrhage Increases Level of Heme Oxygenase-1 and Biliverdin Reductase in the Choroid Plexus.
Front Cell Neurosci. 14: 593305. -
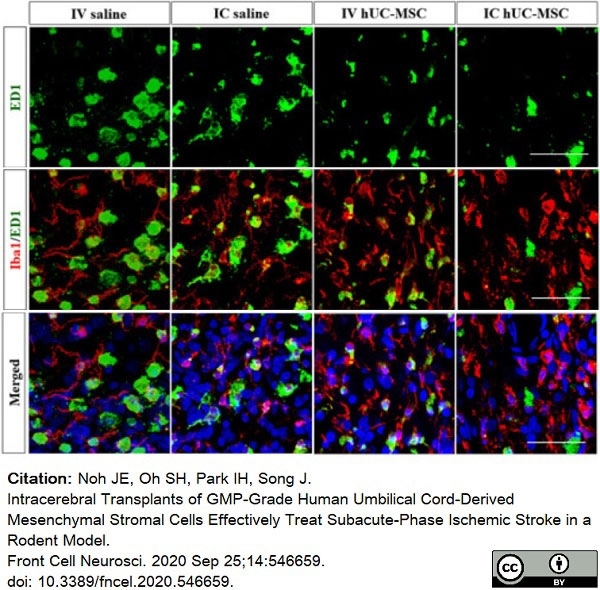
Noh, J.E. et al. (2020) Intracerebral Transplants of GMP-Grade Human Umbilical Cord-Derived Mesenchymal Stromal Cells Effectively Treat Subacute-Phase Ischemic Stroke in a Rodent Model.
Front Cell Neurosci. 14: 546659. -
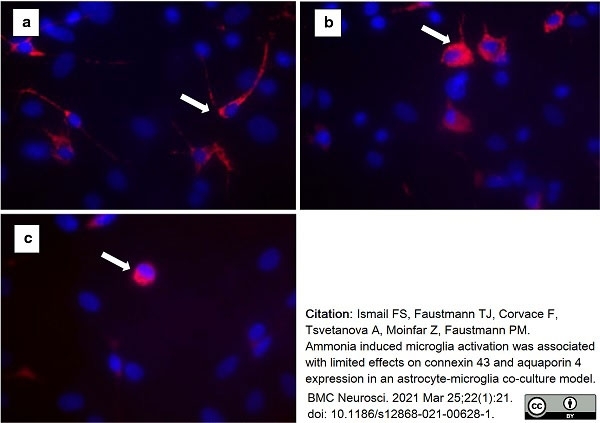
Ismail, S.F. et al. (2021) Ammonia induced microglia activation was associated with limited effects on connexin 43 and aquaporin 4 expression in an astrocyte-microglia co-culture model
BMC Neuroscience. 22 (1): 21. -
Zhao, H.Y. et al. (2020) L-carnitine treatment attenuates renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction.
Korean J Intern Med. 36 (Suppl 1): S180-S195. -
Bennett, M. et al. (2020) Proteoglycan 4 Reduces Neuroinflammation and Protects the Blood-Brain Barrier after Traumatic Brain Injury.
J Neurotrauma. 38 (4): 385-98. -
Wang, Q. et al. (2020) Urinary phosphate-containing nanoparticle contributes to inflammation and kidney injury in a salt-sensitive hypertension rat model.
Commun Biol. 3 (1): 575. -
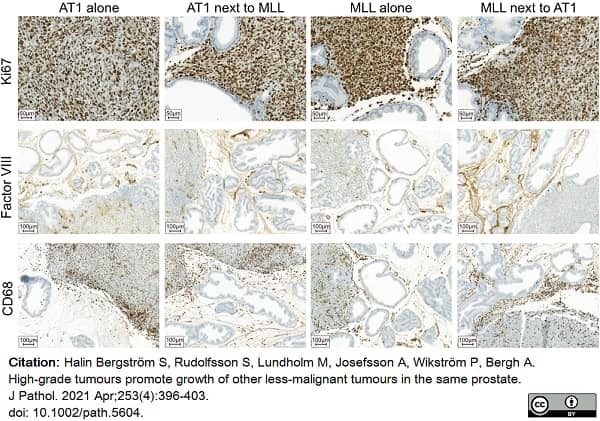
Halin Bergstrom, S. et al. (2021) High-grade tumours promote growth of other less-malignant tumours in the same prostate.
J Pathol. 253 (4): 396-403. -
Kumosa, L.S. & Schouenborg, J. (2021) Profound alterations in brain tissue linked to hypoxic episode after device implantation
Biomaterials. 278: 121143. -
Edanami, N. et al. (2021) Effect of a resin-modified calcium silicate cement on inflammatory cell infiltration and reparative dentin formation after pulpotomy in rat molars.
Aust Endod J. 48 (2): 297-304. -
Anderson, L.E. et al. (2021) Injection of Micronized Human Amnion/Chorion Membrane Results in Increased Early Supraspinatus Muscle Regeneration in a Chronic Model of Rotator Cuff Tear.
Ann Biomed Eng. 49 (12): 3698-710. -
Takagi, M. et al. (2022) Malignant pinealoma observed in the deep cerebral parenchyma of a male Wistar rat.
J Toxicol Pathol. 35 (1): 117-21. -
Hou, Y. et al. (2021) Pseudoginsenoside-F11 promotes functional recovery after transient cerebral ischemia by regulating the microglia/macrophage polarization in rats.
Int Immunopharmacol. 99: 107896. -
Xu, N. et al. (2021) Protective effect and mechanism of rebamipide on NSAIDs associated small bowel injury.
Int Immunopharmacol. 90: 107136. -
Silva, B.A. et al. (2022) Understanding the role of the blood brain barrier and peripheral inflammation on behavior and pathology on ongoing confined cortical lesions.
Mult Scler Relat Disord. 57: 103346. -
Lichtenecker, D.C.K. et al. (2021) Cross-sex testosterone therapy modifies the renal morphology and function in female rats and might underlie increased systolic pressure.
Clin Exp Pharmacol Physiol. 48 (7): 978-86. -
Corvace, F. et al. (2021) Anti-inflammatory properties of lacosamide in an astrocyte-microglia co-culture model of inflammation.
Eur J Pharmacol. 2021, Dec 10: 174696. -
Ingaramo, P.I. et al. (2021) Altered uterine angiogenesis in rats treated with a glyphosate-based herbicide.
Environ Pollut. 296: 118729. -
Pervin, M. et al. (2021) Immunophenotypic analysis of the distribution of hepatic macrophages, lymphocytes and hepatic stellate cells in the adult rat liver.
Anat Histol Embryol. 50 (4): 736-45. -
Leite, A.B. et al. (2021) High-intensity interval training is more effective than continuous training to reduce inflammation markers in female rats with cisplatin nephrotoxicity.
Life Sci. 266: 118880. -
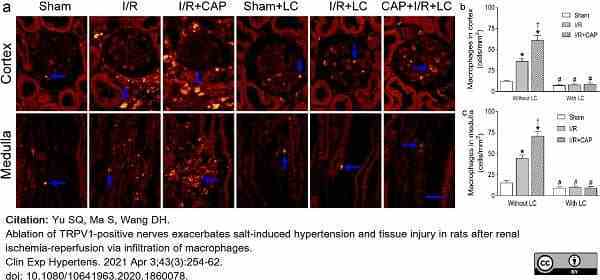
Yu, S.Q. et al. (2021) Ablation of TRPV1-positive nerves exacerbates salt-induced hypertension and tissue injury in rats after renal ischemia-reperfusion via infiltration of macrophages.
Clin Exp Hypertens. 43 (3): 254-262. -
Winkler, A. et al. (2021) Blood-brain barrier resealing in neuromyelitis optica occurs independently of astrocyte regeneration.
J Clin Invest. 131(5):e141694. -
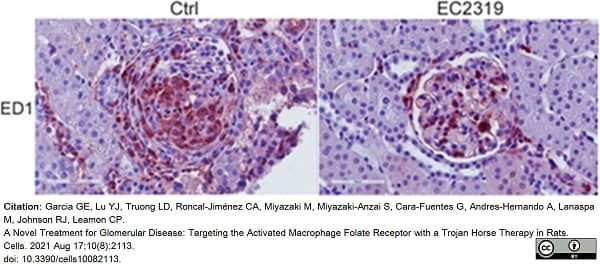
Garcia, G.E. et al. (2021) A Novel Treatment for Glomerular Disease: Targeting the Activated Macrophage Folate Receptor with a Trojan Horse Therapy in Rats.
Cells. 10(8): 2113. -
Pervin, M. et al. (2021) Immunophenotypic analysis of the distribution of hepatic macrophages, lymphocytes and hepatic stellate cells in the adult rat liver.
Anat Histol Embryol. 50 (4): 736-45. -
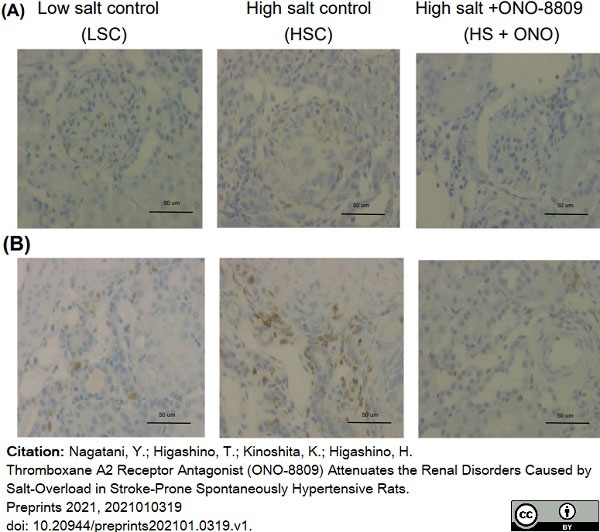
Nagatani, Y. et al. (2021) Thromboxane A2 Receptor Antagonist (ONO-8809) Attenuates the Renal Disorders Caused by Salt-Overload in Stroke-Prone Spontaneously Hypertensive Rats.
Jan 18 [Epub ahead of print]. -
Tong, Y. et al. (2021) The effects of wheel-running using the upper limbs following immobilization after inducing arthritis in the knees of rats.
Physiol Res. 70 (1): 79-87. -
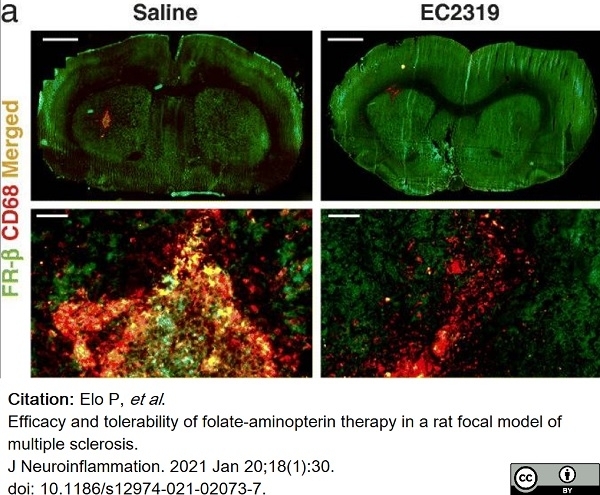
Elo, P. et al. (2021) Efficacy and tolerability of folate-aminopterin therapy in a rat focal model of multiple sclerosis.
J Neuroinflammation. 18 (1): 30. -
Sturgess, D.J. et al. (2021) Left Ventricular Impaired Relaxation and Interstitial Myocarditis Identified in Sepsis-Associated Cardiac Dysfunction: Use of a Rodent Model.
Med Sci Monit. 27: e929512. -
Matsumoto, K. et al. (2021) Role of transient receptor potential vanilloid subtype 2 in lower oesophageal sphincter in rat acid reflux oesophagitis.
J Pharmacol Sci. 146 (3): 125-35. -
Silveira, M.A.D. et al. (2021) Green propolis extract attenuates acute kidney injury and lung injury in a rat model of sepsis.
Sci Rep. 11 (1): 5925. -
Duayer, I.F. et al. (2021) The Protein-Independent Role of Phosphate in the Progression of Chronic Kidney Disease.
Toxins (Basel). 13(7):503. -
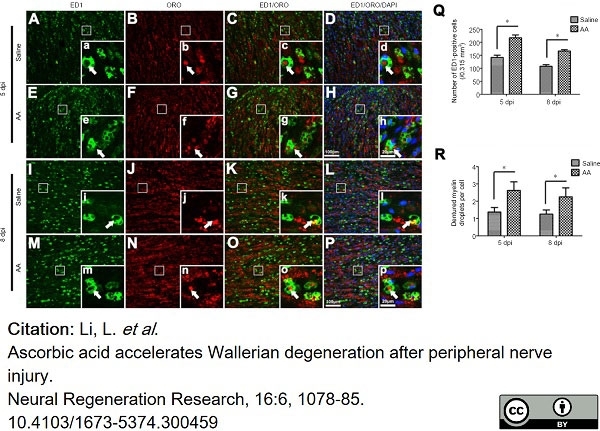
Guo, J. et al. (2021) Ascorbic acid accelerates Wallerian degeneration after peripheral nerve injury
Neural Regeneration Research. 16 (6): 1078. -
Chang, D.J. et al. (2021) Therapeutic Effect of BDNF-Overexpressing Human Neural Stem Cells (F3.BDNF) in a Contusion Model of Spinal Cord Injury in Rats.
Int J Mol Sci. 22 (13): 6970. -
Ziemkiewicz, N. et al. (2022) Laminin-111-Enriched Fibrin Hydrogels Enhance Functional Muscle Regeneration Following Trauma.
Tissue Eng Part A. 28 (7-8): 297-311. -
Matsuyama, S. et al. (2021) Properties of macrophages and lymphocytes appearing in rat renal fibrosis followed by repeated injection of cisplatin.
J Vet Med Sci. 83 (9): 1435-42. -
Dabrowska, S. et al. (2021) Neuroinflammation evoked by brain injury in a rat model of lacunar infarct.
Exp Neurol. 336: 113531. -
Koppe, C. et al. (2021) Local Inflammatory Response after Intramuscularly Implantation of Anti-Adhesive Plasma-Fluorocarbon-Polymer Coated Ti6AI4V Discs in Rats.
Polymers (Basel). 13 (16): 2684 -
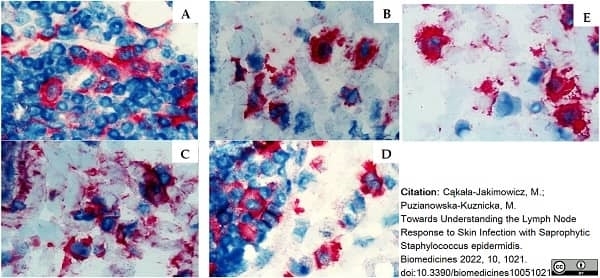
Cąkała-Jakimowicz, M. & Puzianowska-Kuznicka, M. (2022) Towards Understanding the Lymph Node Response to Skin Infection with Saprophytic Staphylococcus epidermidis.
Biomedicines. 10 (5): 1021. -
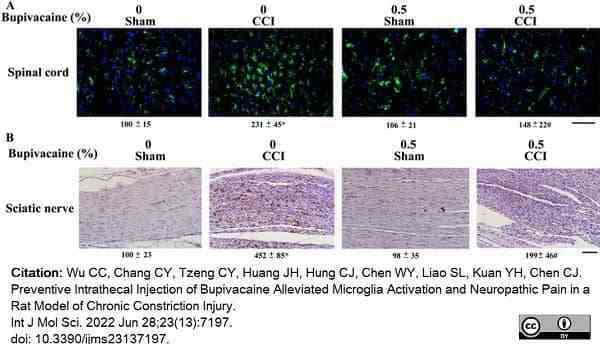
Wu, C.C. et al. (2022) Preventive Intrathecal Injection of Bupivacaine Alleviated Microglia Activation and Neuropathic Pain in a Rat Model of Chronic Constriction Injury.
Int J Mol Sci. 23 (13): 7197. -
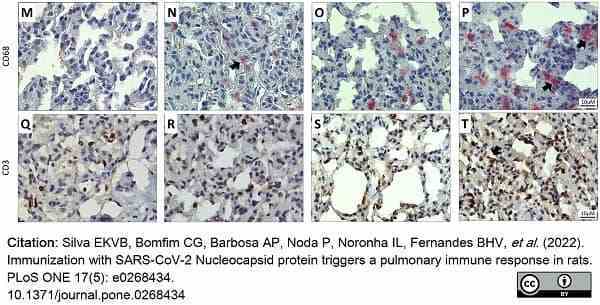
Silva, E.K.V.B. et al. (2022) Immunization with SARS-CoV-2 Nucleocapsid protein triggers a pulmonary immune response in rats.
PLoS One. 17 (5): e0268434. -
Su, H.Y. et al. (2022) Zileuton, a 5-Lipoxygenase Inhibitor, Attenuates Haemolysate-Induced BV-2 Cell Activation by Suppressing the MyD88/NF-κB Pathway.
Int J Mol Sci. 23 (9): 4910. -
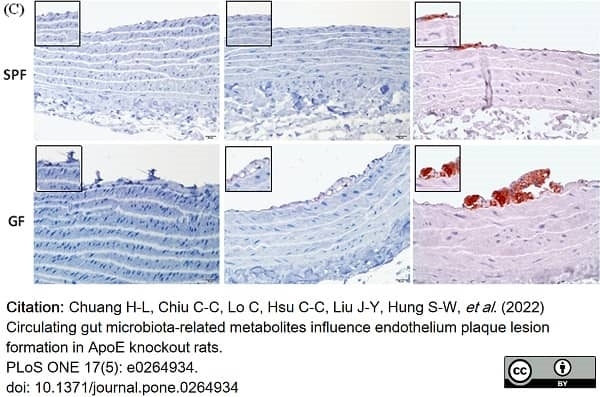
Chuang, H.L. et al. (2022) Circulating gut microbiota-related metabolites influence endothelium plaque lesion formation in ApoE knockout rats.
PLoS One. 17 (5): e0264934. -
Malik, I.A. & Ramadori, G. (2022) Interleukin-6-Production Is Responsible for Induction of Hepatic Synthesis of Several Chemokines as Acute-Phase Mediators in Two Animal Models: Possible Significance for Interpretation of Laboratory Changes in Severely Ill Patients.
Biology (Basel). 11 (3): 470. -
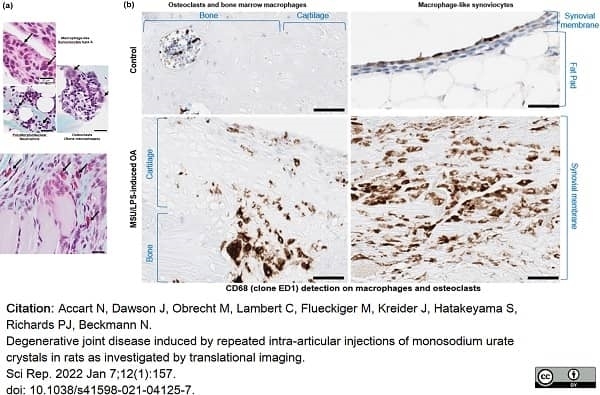
Accart, N. et al. (2022) Degenerative joint disease induced by repeated intra-articular injections of monosodium urate crystals in rats as investigated by translational imaging.
Sci Rep. 12 (1): 157. -
Liu, C.J. et al. (2022) Atorvastatin Decreases Renal Calcium Oxalate Stone Deposits by Enhancing Renal Osteopontin Expression in Hyperoxaluric Stone-Forming Rats Fed a High-Fat Diet.
Int J Mol Sci. 23, 3048. -
Pervin, M. et al. (2022) Possible Cytoprotection of Low Dose Lipopolysaccharide in Rat Thioacetamide-Induced Liver Lesions, Focusing on the Analyses of Hepatic Macrophages and Autophagy.
Toxicol Pathol. 50 (3): 353-65. -
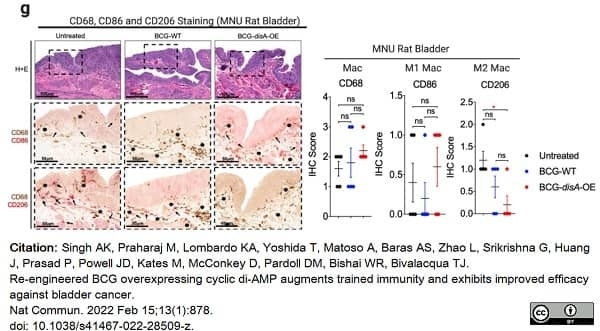
Singh, A.K. et al. (2022) Re-engineered BCG overexpressing cyclic di-AMP augments trained immunity and exhibits improved efficacy against bladder cancer.
Nat Commun. 13 (1): 878. -
Bey, L. et al. (2022) TCDD aggravates the formation of the atherosclerotic plaque in ApoE KO mice with a sexual dimorphic pattern.
Biochimie. 195: 54-8. -
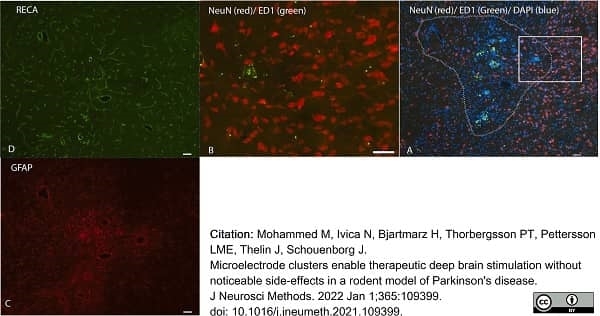
Mohammed, M. et al. (2022) Microelectrode clusters enable therapeutic deep brain stimulation without noticeable side-effects in a rodent model of Parkinson's disease.
J Neurosci Methods. 365: 109399. -
Köhler, R. et al. (2022) Association of systemic antibody response against polyethylene terephthalate with inflammatory serum cytokine profile following implantation of differently coated vascular prostheses in a rat animal model.
J Biomed Mater Res A. 110 (1): 52-63. -
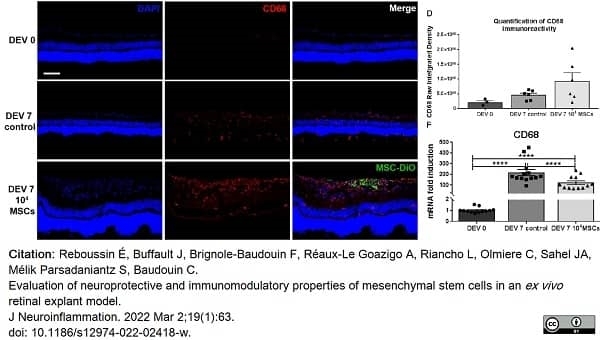
Reboussin, É. et al. (2022) Evaluation of neuroprotective and immunomodulatory properties of mesenchymal stem cells in an ex vivo. retinal explant model.
J Neuroinflammation. 19 (1): 63. -
Hoff, U. et al. (2022) The mTOR inhibitor Rapamycin protects from premature cellular senescence early after experimental kidney transplantation.
PLoS One. 17 (4): e0266319. -
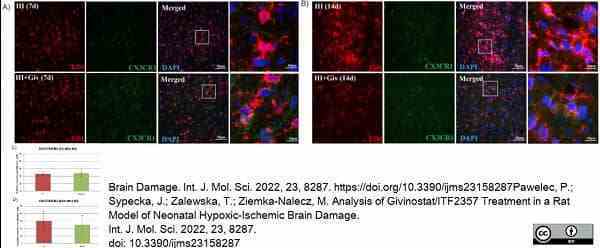
Pawelec, P. et al. (2022) Analysis of Givinostat/ITF2357 Treatment in a Rat Model of Neonatal Hypoxic-Ischemic Brain Damage.
Int J Mol Sci. 23 (15): 8287. -
Merlini, A. et al. (2022) Distinct roles of the meningeal layers in CNS autoimmunity.
Nat Neurosci. 25 (7): 887-99. -
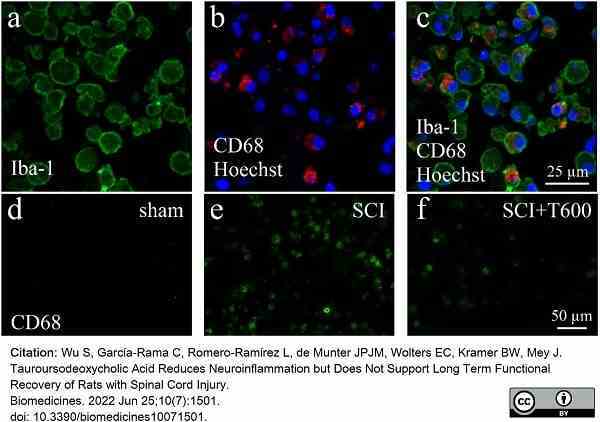
Wu, S. et al. (2022) Tauroursodeoxycholic Acid Reduces Neuroinflammation but Does Not Support Long Term Functional Recovery of Rats with Spinal Cord Injury.
Biomedicines. 10 (7): 1501. -
Ji, Y.B. et al. (2022) Electrostatically optimized adapalene-loaded emulsion for the treatment of acne vulgaris.
Mater Today Bio. 16: 100339. -
Dugbartey, G.J. et al. (2022) Activation of renal CSE/H(2)S pathway by alpha-lipoic acid protects against histological and functional changes in the diabetic kidney.
Biomed Pharmacother. 153: 113386. -
Shvindelman, O. et al. (2022) Harnessing Monocytes for a Liposomal Rosiglitazone-Mediated Anti-Inflammatory Effect
Precision Nanomedicine. 5 (4): 930-945. -
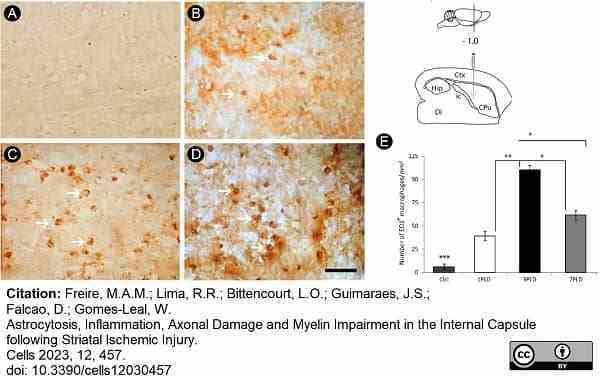
Freire, M.A.M. et al. (2023) Astrocytosis, Inflammation, Axonal Damage and Myelin Impairment in the Internal Capsule following Striatal Ischemic Injury
Cells. 12 (3): 457. -
Oliveira, B.M. et al. (2022) Calcitriol Reduces the Inflammation, Endothelial Damage and Oxidative Stress in AKI Caused by Cisplatin.
Int J Mol Sci. 23 (24): 15877. -
Nürnberger, F. et al. (2022) Systemic Lipopolysaccharide Challenge Induces Inflammatory Changes in Rat Dorsal Root Ganglia: An Ex Vivo Study.
Int J Mol Sci. 23 (21): 13124. -
Yu, M. et al. (2022) Inhibition of Bruton's Tyrosine Kinase Alleviates Monocrotaline-Induced Pulmonary Arterial Hypertension by Modulating Macrophage Polarization.
Oxid Med Cell Longev. 2022: 6526036. -
Shimizu, M.H.M. et al. (2023) Administration of a single dose of lithium ameliorates rhabdomyolysis-associated acute kidney injury in rats.
PLoS One. 18 (2): e0281679. -
Sun, L. et al. (2023) A 3D culture system improves the yield of MSCs-derived extracellular vesicles and enhances their therapeutic efficacy for heart repair.
Biomed Pharmacother. 161: 114557. -
Faustmann, T.J. et al. (2023) Inhibition of Microglial Activation by Amitriptyline and Doxepin in Interferon-β Pre-Treated Astrocyte-Microglia Co-Culture Model of Inflammation.
Brain Sci. 13 (3): 493. -
Ichinohe, N. et al. (2023) CINC-2 and miR-199a-5p in EVs secreted by transplanted Thy1(+) cells activate hepatocytic progenitor cell growth in rat liver regeneration.
Stem Cell Res Ther. 14 (1): 134. -
Forni, M. et al. (2023) Sustained and potent analgesia with negligible side effects enabled by adaptive individualized granular stimulation in rat brainstem.
J Neural Eng. 20: 036014. -
Geng, Y. et al. (2023) Hepatic stellate cells induce an inflammatory phenotype in Kupffer cells via the release of extracellular vesicles.
J Cell Physiol. 238 (10): 2293-303. -
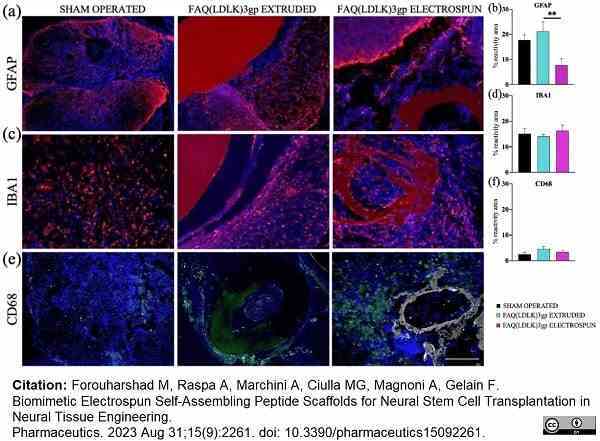
Forouharshad, M. et al. (2023) Biomimetic Electrospun Self-Assembling Peptide Scaffolds for Neural Stem Cell Transplantation in Neural Tissue Engineering.
Pharmaceutics. 15(9):2261. -
Park, C.S. et al. (2023) TRPM7 Mediates BSCB Disruption After Spinal Cord Injury by Regulating the mTOR/JMJD3 Axis in Rats
Mol Neurobiol. Sep 1 [Epub ahead of print]. -
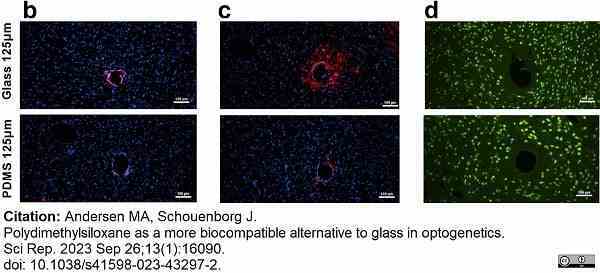
Andersen, M.A. & Schouenborg, J. (2023) Polydimethylsiloxane as a more biocompatible alternative to glass in optogenetics.
Sci Rep. 13 (1): 16090. -
Wang, M. et al. (2024) Casein kinase-2 inhibition promotes retinal ganglion cell survival after acute intraocular pressure elevation.
Neural Regen Res. 19 (5): 1112-8. -
Anderson, L.E. et al. (2023) Bone marrow mobilization & local SDF-1α delivery enhances nascent supraspinatus muscle fiber growth.
Tissue Eng Part A. Oct 28 [Epub ahead of print]. -
Santos, L.F. et al. (2023) Dietary Soy Isoflavones Prevent Metabolic Disturbs Associated with a Deleterious Combination of Obesity and Menopause.
J Med Food. 26 (2): 104-13. -
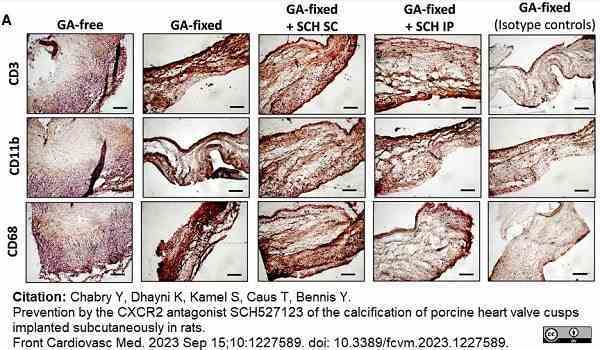
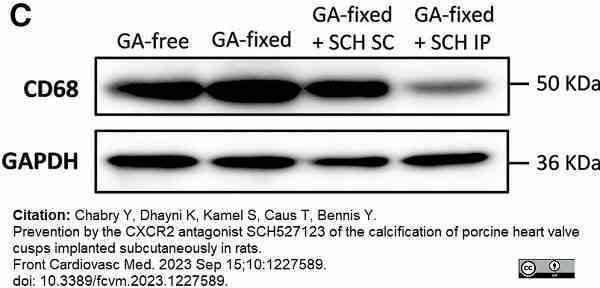
Chabry, Y. et al. (2023) Prevention by the CXCR2 antagonist SCH527123 of the calcification of porcine heart valve cusps implanted subcutaneously in rats.
Front Cardiovasc Med. 10: 1227589. -
Saleh, M.A. et al. (2023) RhoA/ROCK inhibition attenuates endothelin-1-induced glomerulopathy in the rats.
Life Sci. 323: 121687. -
Wenker, S.D. et al. (2023) Microglia-secreted TNF-α affects differentiation efficiency and viability of pluripotent stem cell-derived human dopaminergic precursors.
PLoS One. 18 (9): e0263021. -
Enz, L.S. et al. (2023) An Animal Model for Chronic Meningeal Inflammation and Inflammatory Demyelination of the Cerebral Cortex.
Int J Mol Sci. ;24 (18):13893. -
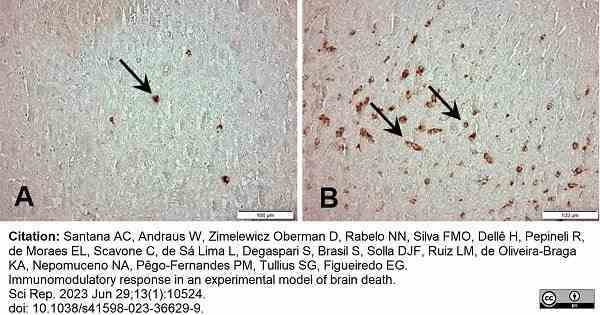
Santana, A.C. et al. (2023) Immunomodulatory response in an experimental model of brain death.
Sci Rep. 13 (1): 10524. -
Maruyama, M. et al. (2023) Liver regeneration after portal vein embolization: comparison between absolute ethanol and N-butyl-cyanoacrylate in an in vivo rat model.
Diagn Interv Radiol. 29 (4): 621-7. -
Du, K. et al. (2023) Pathogenesis of selective damage of granule cell layer in cerebellum of rats exposed to methylmercury.
J Toxicol Sci. 48 (7): 429-39. -
Fanelli, C. et al. (2023) Tamoxifen associated to the conservative CKD treatment promoted additional antifibrotic effects on experimental hypertensive nephrosclerosis.
Sci Rep. 13 (1): 13985. -
De, R.S. et al. (2023) Maternal suboptimal selenium intake and low-level lead exposure affect offspring's microglial immune profile and its reactivity to a subsequent inflammatory hit.
Sci Rep. 13 (1): 21448. -
Xu, Z. et al. (2023) Schwann cells do not promote myogenic differentiation in the EPI loop model.
Tissue Eng Part A. Dec 08 [Epub ahead of print]. -
Dimitrijević, M. et al. (2013) The influence of aging and estradiol to progesterone ratio on rat macrophage phenotypic profile and NO and TNF-α production.
Exp Gerontol. 48 (11): 1243-54. -
Bannerman, D. et al. (2023) Itaconate and citrate releasing polymer attenuates foreign body response in biofabricated cardiac patches
Materials Today Bio. : 100917. -
De Simone, R. et al. (2023) Maternal suboptimal selenium intake and low-level lead exposure affect offspring's microglial immune profile and its reactivity to a subsequent inflammatory hit.
Sci Rep. 13 (1): 21448. -
Zhou, C. et al. (2023) Deletion of mesencephalic astrocyte-derived neurotrophic factor delays and damages the development of white pulp in spleen
Immunobiology. : 152778. -
Zarkesh, I. et al. (2024) ROS scavenging activity of polydopamine nanoparticle-loaded supramolecular gelatin-based hydrogel promoted cardiomyocyte proliferation.
Int J Biol Macromol. 4 Jan: 129228 [Epub ahead of print] -
Nie, R. et al. (2019) Porphyromonas gingivalis Infection Induces Amyloid-β Accumulation in Monocytes/Macrophages.
J Alzheimers Dis. 72 (2): 479-94. -
Alvarez Quintero, G.S. et al. (2024) effects of the mineralocorticoid receptor antagonist eplerenone in experimental autoimmune encephalomyelitis.
J Steroid Biochem Mol Biol. : 106461. -
Huang, C.T. et al. (2020) Melatonin reduces neuropathic pain behavior and glial activation through MT(2) melatonin receptor modulation in a rat model of lysophosphatidylcholine-induced demyelination neuropathy.
Neurochem Int. 140: 104827. -
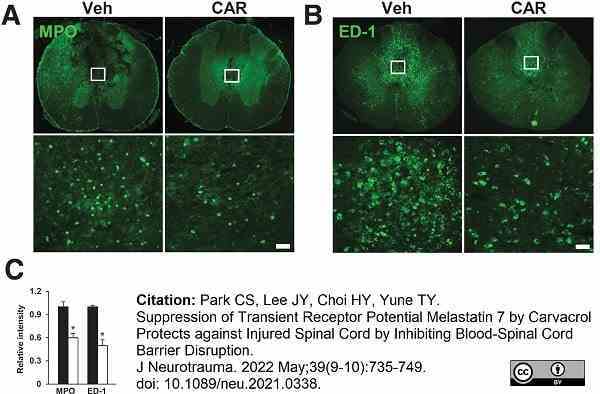
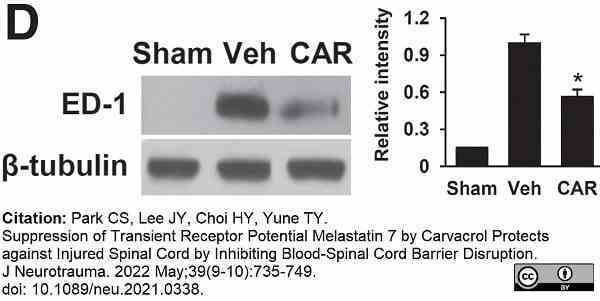
Park, C.S. et al. (2022) Suppression of Transient Receptor Potential Melastatin 7 by Carvacrol Protects against Injured Spinal Cord by Inhibiting Blood-Spinal Cord Barrier Disruption.
J Neurotrauma. 39 (9-10): 735-49. -
Supasai, S. et al. (2020) Acute administration of diazepam or midazolam minimally alters long-term neuropathological effects in the rat brain following acute intoxication with diisopropylfluorophosphate.
Eur J Pharmacol. 886: 173538. -
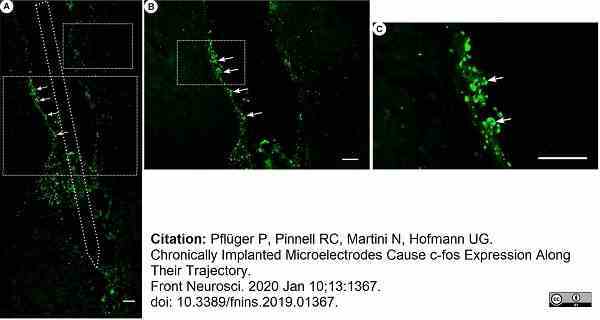
Pflüger, P. et al. (2019) Chronically Implanted Microelectrodes Cause c-fos Expression Along Their Trajectory.
Front Neurosci. 13: 1367. -
Huang, C.T. et al. (2020) Glycemic control with insulin attenuates sepsis-associated encephalopathy by inhibiting glial activation via the suppression of the nuclear factor kappa B and mitogen-activated protein kinase signaling pathways in septic rats.
Brain Res. 1738: 146822. -
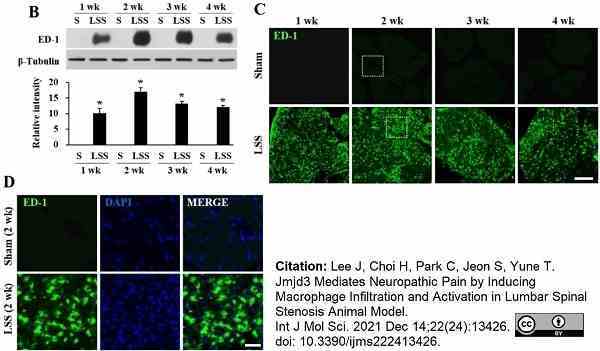
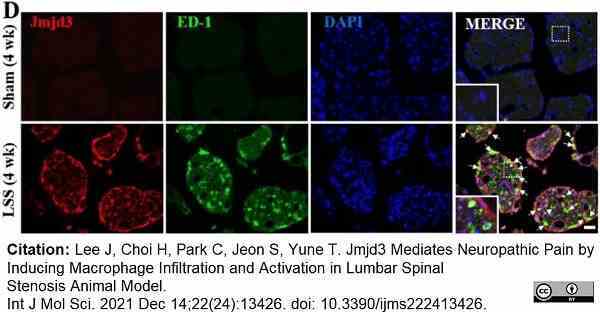
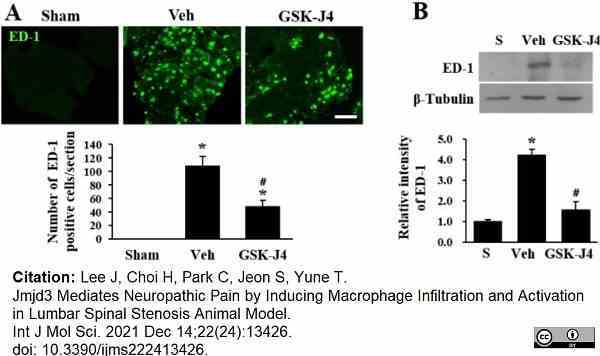
Lee, J. et al. (2021) Jmjd3 Mediates Neuropathic Pain by Inducing Macrophage Infiltration and Activation in Lumbar Spinal Stenosis Animal Model.
Int J Mol Sci.22 (24): 13426. -
Anttila, J.E. et al. (2024) MANF protein expression is upregulated in immune cells in the ischemic human brain and systemic recombinant MANF delivery in rat ischemic stroke model demonstrates anti-inflammatory effects.
Acta Neuropathol Commun. 12 (1): 10. -
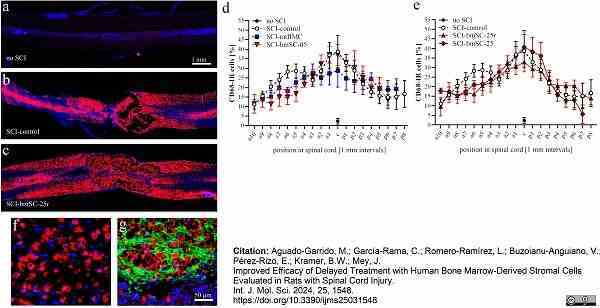
Aguado-Garrido, M. et al. (2024) Improved Efficacy of Delayed Treatment with Human Bone Marrow-Derived Stromal Cells Evaluated in Rats with Spinal Cord Injury
Int J Mol Sci. 25 (3): 1548. -
Xu, Z. et al. (2024) Schwann Cells Do Not Promote Myogenic Differentiation in the EPI Loop Model.
Tissue Eng Part A. Jan 11 [Epub ahead of print]. -
Elmounedi, N. et al. (2024) Ozone therapy (O(2)-O(3)) alleviates the progression of early intervertebral disc degeneration via the inhibition of oxidative stress and the interception of the PI3K/Akt/NF-κB signaling pathway.
Int Immunopharmacol. 129: 111596. -
Xu, Q. & Cheung, F.T.R. (2024) Melatonin at repeated doses alleviates hyperglycemia-exacerbated cerebral ischemia-reperfusion injury at 72 h via anti-inflammation and anti-apoptosis.
IBRO Neuroscience Reports. 5 Mar [Epub ahead of print]. -
Martinez, M.E. et al. (2020) Cotton Rat Placenta Anatomy and Fc Receptor Expression and Their Roles in Maternal Antibody Transfer.
Comp Med. 70 (6): 510-9. -
Cardoso, C.S. et al. (2024) New approaches to second-degree burn healing: Polyvinyl alcohol membrane loaded to arnica combined to laser therapy.
J Biomater Appl. : 8853282241238609 [Epub ahead of print] -
Xu, Q. & Cheung, F.T.R. (2024) Melatonin at repeated doses alleviates hyperglycemia-exacerbated cerebral ischemia-reperfusion injury at 72 h via anti-inflammation and anti-apoptosis
IBRO Neuroscience Reports. 16: 418-27. -
Hullugundi,.S.K. et al. (2024) Cholesterol-dependent LXR transcription factor activity represses pronociceptive effects of estrogen in sensory neurons and pain induced by myelin basic protein fragments
Brain, Behavior, & Immunity - Health. : 100757.
- Synonyms
- ED1
- RRID
- AB_2291300
- UniProt
- Q4FZY1
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Rat ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up