Neuroinflammation, Microglia, and Alzheimer’s
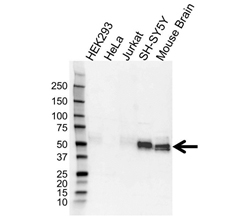
Detection of tau with Rabbit Anti-Microtubule Associated Protein, tau (VPA00677) revealed by HRP conjugated Goat Anti-Rabbit IgG (STAR208P) and visualized on the ChemiDoc MP Imaging System.
Introduction
Neuroinflammation, as its name indicates, is the inflammatory response within the brain or spinal cord. While it primarily carries out a protective function in the brain, once it reaches chronic levels neuronal damage ensues. Alzheimer's disease (AD) is an example of excessive neuroinflammation.
This process is driven by the immune cells of the central nervous system (CNS); these are the resident microglia and astrocytes, and endothelial cells. If the blood brain-barrier (BBB) has become compromised through prolonged and systemic inflammation, peripherally derived immune cells can also enter the brain. Cytokines, chemokines, reactive oxygen species, and other secondary messengers are the mediators of neuroinflammation.
Glial Cells
Microglia are an ontogenically distinct population, separate from the peripheral mononuclear phagocyte system. Embryonic yolk sac myeloid precursor cells migrate to the future CNS area around day 8.5 of development (Ginhoux et al. 2010). They share the same progenitor as long-lived tissue macrophages (Alliot et al. 1999). This makes microglia long lived cells that are not replaced by emigrant myeloid cells from the bone marrow but can be generated from nestin-positive cells in the CNS that differentiate into microglia (Ajami et al. 2007, Elmore et al. 2014). This low turnover, characteristic of microglia, has been suggested to make them liable to a stress or damage induced proinflammatory profile. However, under pathological conditions peripheral monocytes/macrophages can infiltrate the CNS and amplify the deleterious neuroinflammatory response.
Glial Cell Function
Microglia have been assigned wide ranging functions from embryonic development to adult homeostasis, as well as a range of pathologic profiles. How they manage these contrasting functions has not been fully resolved. It is not only during development that microglia modulate neuronal numbers using cell death (Antony et al. 2011, Tong and Vidyadaran 2016) and proliferation and survival support (Ribeiro Xavier et al. 2015), but they also promote (Rogers et al. 2011) and repress (Vukovic et al. 2012) neurogenesis in the adult brain. In the latter, this is achieved via the chemokine fractalkine (CX3CL1) binding to the CX3C chemokine receptor 1 (CX3CR1) on microglia to promote neurogenesis. When CX3CL1 levels decline, for example in advancing age, neurogenesis is inhibited.
Microglia regulate the number of synapses in the developing and adult brain by either stripping (Kettenmann et al. 2013) or stabilizing (Ikegami et al. 2019) dendritic spines and inhibitory synapses. It is thought that distinct mechanisms exist for these two processes (Parkhurst et al. 2013, Vilalta and Brown 2018).
Additionally, there is cross-talk between microglia and astrocytes leading to cross-regulation of functions by these two CNS cell types (Jha et al. 2019). Data suggest that, at least in the case of traumatic brain injury (TBI), microglia, by downregulating expression of the P2Y1 purinergic receptor, transform reactive astrocytes into a neuroprotective phenotype (Shinozaki et al. 2017). Other work has implicated microglia in angiogenesis (Zhao et al. 2018), and put forward that dysregulation of microglia results in neurovascular dysfunction which promotes Alzheimer's disease (AD) (Singh-Bains et al. 2019). This leads to the strong suggestion that that microglia exist as distinct subclasses (Table 1, modified from Stratoulias et al. 2019).
Table 1. Proposed microglial subtypes.
Name |
Satellite Microglia |
KSPG-microglia |
Microglia Supporting Neurogenesis |
Hox8b-microglia |
CD11c-microglia |
Dark Microglia |
|---|---|---|---|---|---|---|
|
Function |
Interact with the axon initial segment (AIS) of neurons in the healthy brain. Loss of interaction upon injury |
Typical appearance upon different insults, i.e. around motor neurons in ALS; away from brain trauma |
Essential for neuroblast survival and migration in SVZ/RMS |
Absence of Hoxb8 function in microglia impacts on the corticostriatal neuronal circuit and leads to impaired grooming, anxiety, and social behaviors |
Promote myelination and neurogenesis in the neonatal brain |
Interact with blood vessels and synapses |
|
Characteristics |
Localized to the axonal side of the neuron's cell body. Exhibit a single process overlapping with the AIS |
Keratan sulfate positive cells |
IBA1–, isolectin B4–, CD68-negative, P2RY12low, |
YFP-Hoxb8-expressing cells |
CD11c-positive, Igf1-producing cells |
Appear as dark by electron microscopy |
|
Key markers |
CX3CR1 |
Neuroinflammation and Glial Cells in Alzheimer's Disease
In AD, not only the neuronal component contributes to the disease, but the immune system also drives the pathology. Neuroinflammation, caused by Aβ plaques and tau tangles, is just as damaging to the brain as the filaments themselves (Zhang et al. 2013). The involvement of the immune compartment is also shown by the association of a TREM2 variant and CD33 with AD. For TREM2, the association is linked to a reduced function mutant (Guerreiro et al. 2013), and conversely for CD33, the increased expression links to AD (Griciuc et al. 2013).
Microglia play the major role in neuroinflammation driven by cytokines and chemokines. Reactive microglia secrete proinflammatory cytokines like interleukin-1beta (IL-1β), IL-6, IL-18, and tumor necrosis factor-alpha (TNF-α). Expression of the CCL2, CCR3, and CCR5 chemokines is upregulated, leading to local inflammatory responses that create a neurotoxic environment (Heneka et al. 2015). In the AD pathology, microglial stimulation by Aβ oligomers and plagues drives neuroinflammation, via innate immune receptors such as the Toll-like receptors (TLRs), the LPS receptor CD14, scavenger receptors like CD36, CD47, and scavenger receptor type A (CD204) (Fassbender et al. 2004, El Khoury et al. 2003, Frenkel et al. 2013). CD36 interacts with Aβ, leading to the activation of the NACHT, LRR, and PYD domains-containing protein 3 (NLRP3) inflammasome and the release of proinflammatory IL-1β, thus sustaining neuroinflammation (Doens et al. 2017).
Much remains to be discovered about the regulation of neuroinflammation and microglial activation. In particular as early microglial activation in AD supports Aβ clearance and is neuroprotective. It is later in AD that Aβ aggregation and proinflammatory cytokines lead to a reduction in Aβ clearance and neurodegeneration (Hickman et al. 2008).
Bio-Rad has a long and successful track record as a supplier of innate immune system antibodies with a focus on inflammation. Discover Bio-Rad's antibody offering for immunology and neurological diseases.
References
- Ajami B et al. (2007). Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 10, 1,538-1,543.
- Alliot F. et al. (1999). Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res. Dev. Brain Res. 117, 145-152.
- Antony JM et al. (2011). Endogenous microglia regulate development of embryonic cortical precursor cells. J. Neurosci. Res. 89(3), 286-298.
- Doens D et al. (2017). Identification of inhibitors of CD36-amyloid Beta binding as potential agents for Alzheimer's disease. ACS Chem Neurosci. 8(6), 1,232-1,241.
- El Khoury JB et al. (2003). CD36 mediates the innate host response to beta-amyloid. J. Exp. Med. 197, 1,657-1,666.
- Elmore MR et al. (2014). Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82, 380-397.
- Fassbender K. et al. (2004). The LPS receptor (CD14) links innate immunity with Alzheimer’s disease. FASEB J. 18, 203-205.
- Frenkel D et al. (2013). Scara1 deficiency impairs clearance of soluble amyloid-β by mononuclear phagocytes and accelerates Alzheimer's-like disease progression. Nat Commun. 4, 2,030.
- Ginhoux F et al. (2010). Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841-845.
- Griciuc A et al. (2013). Alzheimer's disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 78(4), 631-643.
- Guerreiro R et al. (2013). TREM2 variants in Alzheimer's disease. N Engl J Med. 368(2), 117-127.
- Heneka MT et al. (2015). Neuroinflammation in Alzheimer's disease. Lancet Neurol. 14(4), 388-405.
- Hickman SE et al. (2008). Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer's disease mice. J Neurosci. 28(33), 8,354-8,360.
- Kettenmann H et al. (2013). Microglia: new roles for the synaptic stripper. Neuron. 77, 10-18.
- Ikegami A et al. (2019). Microglia: lifelong modulator of neural circuits. Neuropathology 39, 173-80.
- Jha MK et al. (2019). Microglia-astrocyte crosstalk: an intimate molecular conversation. Neuroscientist. 25, 227-240
- Parkhurst CN et al. (2013). Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 155, 1,596-1,609.
- Ribeiro Xavier AL et al. (2015). A distinct population of microglia supports adult neurogenesis in the subventricular zone. J Neurosci. 35(34), 11848-11861.
- Rogers JT et al. (2011). CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J Neurosci. 31, 16,241-16,250.
- Singh-Bains MK et al. (2019). Altered microglia and neurovasculature in the Alzheimer’s disease cerebellum. Neurobiol Dis. 132, 104,589.
- Shinozaki Y et al. (2017). Transformation of astrocytes to a neuroprotective phenotype by microglia via P2Y1 receptor downregulation. Cell Rep. 19, 1,151-1,164.
- Stratoulias V et al. (2019). Microglial subtypes: diversity within the microglial community. EMBO J. 38, e101997.
- Tong CK and Vidyadaran S (2016). Role of microglia in embryonic neurogenesis. Exp. Biol. Med. 241(15), 1,669-1,675.
- Vilalta A and Brown GC. (2018). Neurophagy, the phagocytosis of live neurons and synapses by glia, contributes to brain development and disease. FEBS J. 285, 3,566-3,575
- Vukovic J et al. (2012). Microglia modulate hippocampal neural precursor activity in response to exercise and aging. J Neurosci. 32, 6,435-6,443.
- Zhang B. et al. (2013). Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer's disease. Cell. 153(3), 707-720.
- Zhao X et al. (2018). Microglial interactions with the neurovascular system in physiology and pathology. Dev Neurobiol. 78, 604-617.



