T Cells in Neuroinflammation

- On This Page
- Neuroinflammation Triggers
- T Cells
- Tregs
- CD8 T Cells
- References
Sign up to Our Emails

Be the first to know when we launch new products and resources to help you achieve more in the lab.
s
Neuroinflammation Triggers
Neurodegenerative diseases, such as Alzheimer's disease (AD), are caused by a neuronal component interacting with immunological mechanisms in the brain.
Misfolded and aggregated proteins, such as amyloid protein in AD, trigger pattern recognition receptors on microglia, leading to an innate immune response accompanied by secretion of inflammatory mediators. The adaptive immune compartment, mainly T cells, also contributes to the pathology of neurodegenerative disease by modulating neuroinflammation. This is generally a beneficial process to reestablish homeostasis in the brain. However, when acute inflammatory responses turn chronic, further brain injury is caused.
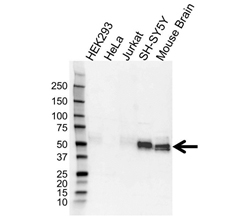
Detecting tau.
Fig. 1. Western blot analysis of tau with Rabbit Anti-Microtubule Associated Protein tau (VPA00677), followed by HRP conjugated Goat Anti-Rabbit IgG (STAR208P) and visualized on the ChemiDoc MP Imaging System.
T Cells
While microglia have received more attention, T cells were associated with Alzheimer's disease soon after the identification of amyloid-beta deposits (Glenner and Wong 1984) and intraneuronal neurofibrillary tangles consisting of tau (Goedert et al.1992) (Figure 1), as the main pathological identifiers of AD. The first reports associating T cells with AD were published in the 1980s (Rogers et al.1988, McGeer et al.1989).
Subsequent discoveries of vascular and parenchymal T cells in post-mortem brains strengthened the evidence that T cells are involved with neuroinflammation in AD (Giubilei et al. 2003, Zhang et al. 2003, Ferretti et al. 2016, Merlini et al. 2018). Data also became available on the possible roles of T helper 1 and 2 (Th1 and Th2) cells. An experiment using glial cells as antigen presenting cells suggested that amyloid beta specific Th1 cells induce the secretion of proinflammatory cytokines by microglial cells, whereas amyloid beta specific Th2 cells suppress proinflammatory IFN-gamma production by Th1 cells and IL-17 production by Th17 cells (McQuillan et al. 2010). More recently, T regulatory cells (Treg) have been examined for their role in AD (Dansokh et al. 2016).
Tregs
The key function of Tregs is to suppress inflammation. In the context of neuroinflammation, they have been proposed to increase survival in amyotrophic lateral sclerosis (ALS) (Henkel et al. 2013), modulate microglial inflammatory responses (Xie et al. 2015), and protect dopaminergic neurons in a mouse model of Parkinson’s disease (Huang et al. 2020). Tregs from ALS patients were found to be deficient at suppressing responder T lymphocyte proliferation (Beers et al. 2017).
Another study isolated Tregs from patients with AD, individuals with mild cognitive impairment, and healthy controls. Then it determined the Tregs phenotype with multicolor flow cytometry (CD3, CD8, CD4, CD25, CD73, and PD-1) and assayed their ability to suppress lymphocyte proliferation. T cell populations were then expanded in vitro and the lymphocyte suppression assay was repeated. Tregs from AD patients had reduced CD25 expression and much lower ability to suppress lymphocyte proliferation. However, after Treg expansion, this ability increased in all samples, hinting at a possible method for modulating the inflammatory mode of Alzheimer’s disease (Faridar et al. 2020).
CD8 T Cells
CD8+ T cells were first detected in the brains of AD patients in the early 2000s and their role in AD is less well defined (Togo et al. 2002). Recent work comparing samples from AD patients and healthy controls revealed higher numbers of activated HLA-DR+ CD8+ T cells and greater production of pro-inflammatory cytokines by cytotoxic T cells in the cerebrospinal fluid (CSF) of AD patients, compared with healthy controls. These cytotoxic cells were CD3+CD8+CD27− T effector memory CD45RA+ (TEMRA) cells and their increase was associated with lower cognition. The increase in cell number was clonal, with a specificity to two Epstein-Barr virus (EBV) antigens (Gate et al. 2020). While there was an association between AD and the clonal expansion of EBV-specific TCRs in CSF from patients with AD, Gate et al. caution that these data are not evidence of a causal link between EBV infectivity and AD.
Further work remains to be done on the role of CD8+ T cells in AD. Other aspects for further investigation are the pro-inflammatory nature of CD8+ TEMRA cells, driven by IFN-gamma secretion during active proliferation (Verma et al. 2017). IFN-gamma enhances neuronal killing by CD8+ T cells via the FasR-FasL pathway. Additionally, overexpression of MHC-I on neurons due to IFN-gamma exposure was found to lead to neuronal damage by CD8+ T cells via TCR-MHC-I binding; MHC-I has been detected on neurons in hippocampal and cortical brain regions, both areas that suffer heavy damage in AD (Cebrián et al. 2014).
Bio-Rad has a long and successful track record as a supplier of adaptive and innate immune system antibodies with a focus on inflammation. Discover Bio-Rad's antibody offering for immunology and neurological diseases.
References
- Beers DR et al. (2017). ALS patients' regulatory T lymphocytes are dysfunctional, and correlate with disease progression rate and severity. JCI Insight 2, e89530.
- Cebrián C et al. (2014). Neuronal MHC-I expression and its implications in synaptic function, axonal regeneration and Parkinson's and other brain diseases. Front Neuroanat 8, 114.
- Dansokh C et al. (2016). Regulatory T cells delay disease progression in Alzheimer-like pathology. Brain 139, 1237–1251.
- Faridar A et al. (2020). Restoring regulatory T-cell dysfunction in Alzheimer's disease through ex vivo expansion. Brain Commun 2, fcaa112.
- Ferretti MT et al. (2016). T-cell brain infiltration and immature antigen-presenting cells in transgenic models of Alzheimer's disease-like cerebral amyloidosis. Brain Behav Immun 54, 211–225.
- Gate D et al. (2020). Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer's disease. Nature 577, 399–404.
- Giubilei F et al. (2003). T cell response to amyloid-beta and to mitochondrial antigens in Alzheimer's disease. Dement Geriatr Cogn Disord 16, 35–38.
- Glenner GG and Wong C W (1984). Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 120, 885–890.
- Goedert M et al. (1992). Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms. Neuron 8, 159–168.
- Henkel JS et al. (2013). Regulatory T-lymphocytes mediate amyotrophic lateral sclerosis progression and survival. EMBO Mol Med 5, 64–79.
- Huang Y et al. (2020). Treg cells attenuate neuroinflammation and protect neurons in a mouse model of Parkinson's disease. J Neuroimmune Pharmacol 15, 224–237.
- Merlini M et al. (2018). Extravascular CD3+ T cells in brains of Alzheimer disease patients correlate with tau but not with amyloid pathology: an immunohistochemical study. Neurodegener Dis 18, 49–56.
- McGeer PL et al. (1989). Immune system response in Alzheimer's disease. Can J Neurol Sci 16, 516–527.
- McQuillan K et al. (2010). Activation of mixed glia by Abeta-specific Th1 and Th17 cells and its regulation by Th2 cells. Brain Behav Immun 24, 598–607.
- Rogers J et al. (1988). Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer's disease. Neurobiol Aging 9, 339–349.
- Togo T et al. (2002). Occurrence of T cells in the brain of Alzheimer's disease and other neurological diseases. J Neuroimmunol 124, 83–92.
- Verma K et al. (2017). Human CD8+ CD57- TEMRA cells: Too young to be called "old". PloS One 12, e0177405.
- Zhang J et al. (2003). Telomere dysfunction of lymphocytes in patients with Alzheimer disease. Cogn Behav Neurol 16, 170–176.
- Xie L et al. (2015). Cerebral regulatory T cells restrain microglia/macrophage-mediated inflammatory responses via IL-10. Eur J Immunol 45, 180–191.


