IL-10 antibody | CC318



Mouse anti Bovine Interleukin-10
- Product Type
- Monoclonal Antibody
- Clone
- CC318
- Isotype
- IgG2b
- Specificity
- IL-10
| Mouse anti Bovine interleukin-10 antibody, clone CC318 recognizes bovine IL-10. Mouse anti Bovine interleukin-10 antibody, clone CC318 has been shown not to inhibit the biological activity of IL-10. |
- Target Species
- Bovine
- Species Cross-Reactivity
-
Target Species Cross Reactivity Horse Sheep - N.B. Antibody reactivity and working conditions may vary between species.
- Product Form
- Purified IgG - liquid
- Preparation
- Purified IgG prepared by affinity chromatography on Protein A from tissue culture supernatant
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
- 0.09% sodium azide (NaN3)
- Carrier Free
- Yes
- Immunogen
- Plasmid cDNA encoding bovine IL-10.
- Approx. Protein Concentrations
- IgG concentration 1.0 mg/ml
- Fusion Partners
- Spleen cells from immunized Balb/c mice were fused with cells of the mouse SP2/0 myeloma cell line
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| ELISA | 5ug/ml | 10ug/ml | |
| ELISpot | |||
| Flow Cytometry 1 |
- 1 Membrane permeabilization is required for this application. The use of Leucoperm (Product Code BUF09) is recommended for this purpose.
- ELISA
- Mouse anti Bovine interleukin-10 antibody, clone CC318 may be used as a capture antibody in a sandwich ELISA assay for bovine IL-10 in combination with MCA2111B as detection reagent (Bannermann et al. 2004).
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Mouse IgG2b Negative Control | MCA691 | F | 100 Tests |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Mouse IgG2b Negative Control | ||||||
References for IL-10 antibody
-
Kwong, L.S. et al. (2002) Development of an ELISA for bovine IL-10.
Vet Immunol Immunopathol. 85 (3-4): 213-23. -
Bannerman, D.D. et al. (2004) Escherichia coli and Staphylococcus aureus elicit differential innate immune responses following intramammary infection.
Clin Diagn Lab Immunol. 11: 463-72. -
Abbott, J.R. et al. (2005) Rapid and long-term disappearance of CD4+ T lymphocyte responses specific for Anaplasma marginale major surface protein-2 (MSP2) in MSP2 vaccinates following challenge with live A. marginale.
J Immunol. 174: 6702-15. -
Berger, S.T. and Griffin, F.T. (2006) A comparison of ovine monocyte-derived macrophage function following infection with Mycobacterium avium ssp. avium and Mycobacterium avium ssp. paratuberculosis.
Immunol Cell Biol. 84: 349-56. -
Davis, T.L. and Pate, J.L. (2007) Bovine luteal cells stimulate proliferation of major histocompatibility nonrestricted gamma delta T cells.
Biol Reprod. 77: 914-22. -
Denis, M. et al. (2007) Enhancement of the sensitivity of the whole-blood gamma interferon assay for diagnosis of Mycobacterium bovis infections in cattle.
Clin Vaccine Immunol. 14 (11): 1483-9. -
Hamza, E. et al. (2007) Modulation of allergy incidence in icelandic horses is associated with a change in IL-4-producing T cells.
Int Arch Allergy Immunol. 144: 325-37. -
Souza, M. et al. (2008) Pathogenesis and immune responses in gnotobiotic calves after infection with the genogroup II.4-HS66 strain of human norovirus.
J Virol. 82: 1777-86.
View The Latest Product References
-
Flynn, R.J. et al. (2008) Possible role for Toll-like receptors in interaction of Fasciola hepatica excretory/secretory products with bovine macrophages.
Infect Immun. 76: 678-84. -
Weiss DJ et al. (2008) Bovine monocyte TLR2 receptors differentially regulate the intracellular fate of Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium.
J Leukoc Biol. 83 (1): 48-55. -
Scandurra, G.M. et al. (2009) Assessment of live candidate vaccines for paratuberculosis in animal models and macrophages.
Infect Immun. 78: 1383-9. -
Olivier, M. et al. (2009) Capacities of migrating CD1b+ lymph dendritic cells to present Salmonella antigens to naive T cells.
PLoS One. 7: e30430. -
Jones, G.J. et al. (2010) Simultaneous measurement of antigen-stimulated interleukin-1 beta and gamma interferon production enhances test sensitivity for the detection of Mycobacterium bovis infection in cattle.
Clin Vaccine Immunol. 17: 1946-51. -
Coad, M. et al. (2010) Repeat tuberculin skin testing leads to desensitisation in naturally infected tuberculous cattle which is associated with elevated interleukin-10 and decreased interleukin-1 beta responses.
Vet Res. 41: 14. -
Ferret-Bernard, S. et al. (2010) Cellular and molecular mechanisms underlying the strong neonatal IL-12 response of lamb mesenteric lymph node cells to R-848.
PLoS One. 5: e13705. -
Parker, D.G. et al. (2010) Lentivirus-mediated gene transfer of interleukin 10 to the ovine and human cornea.
Clin Experiment Ophthalmol. 38: 405-13. -
Rinaldi, M. et al (2010) A sentinel function for teat tissues in dairy cows: dominant innate immune response elements define early response to E. coli mastitis.
Funct Integr Genomics. 10: 21-38. -
Wenz, J.R. et al. (2010) Factors associated with concentrations of select cytokine and acute phase proteins in dairy cows with naturally occurring clinical mastitis.
J Dairy Sci. 93: 2458-70. -
den Hartog, G. et al. (2011) Modulation of human immune responses by bovine interleukin-10.
PLoSone 6: e18188 -
Shu, D. et al. (2011) Diverse cytokine profile from mesenteric lymph node cells of cull cows severely affected with Johne's disease.
Clin Vaccine Immunol. 18: 1467-76. -
Ferret-Bernard, S. et al. (2011) Mesenteric lymph node cells from neonates present a prominent IL-12 response to CpG oligodeoxynucleotide via an IL-15 feedback loop of amplification.
Vet Res. 42:19. -
Ikebuchi, R. et al. (2013) Blockade of bovine PD-1 increases T cell function and inhibits bovine leukemia virus expression in B cells in vitro.
Vet Res. 44: 59. -
McGill, J.L. et al. (2013) Differential chemokine and cytokine production by neonatal bovine γ/δ T-cell subsets in response to viral toll-like receptor agonists and in vivo respiratory syncytial virus infection.
Immunology. 139: 227-44. -
Redondo, E. et al. (2014) Induction of interleukin-8 and interleukin-12 in neonatal ovine lung following experimental inoculation of bovine respiratory syncytial virus.
J Comp Pathol. 150 (4): 434-48. -
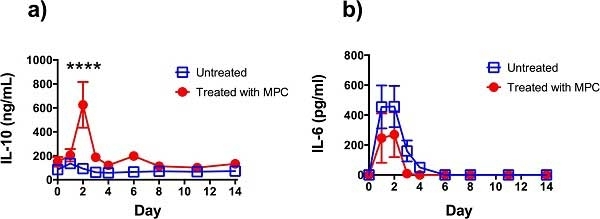
Dooley LM et al. (2015) Effect of mesenchymal precursor cells on the systemic inflammatory response and endothelial dysfunction in an ovine model of collagen-induced arthritis.
PLoS One. 10 (5): e0124144. -
Rainard, P. et al. (2016) Innate and Adaptive Immunity Synergize to Trigger Inflammation in the Mammary Gland.
PLoS One. 11 (4): e0154172. -
Pomeroy B et al. (2016) Impact of in vitro treatments of physiological levels of estradiol and progesterone observed in pregnancy on bovine monocyte-derived dendritic cell differentiation and maturation.
Vet Immunol Immunopathol. 182: 37-42. -
Rodrigues, V. et al. (2017) Development of a bead-based multiplexed assay for simultaneous quantification of five bovine cytokines by flow cytometry.
Cytometry A. 91 (9): 901-7. -
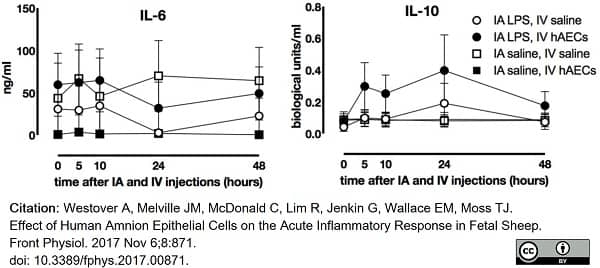
Westover, A. et al. (2017) Effect of Human Amnion Epithelial Cells on the Acute Inflammatory Response in Fetal Sheep.
Front Physiol. 8: 871. -
Canal AM et al. (2017) Immunohistochemical detection of pro-inflammatory and anti-inflammatory cytokines in granulomas in cattle with natural Mycobacterium bovis infection.
Res Vet Sci. 110: 34-39. -
Cassady-Cain, R.L. et al. (2017) Inhibition of Antigen-Specific and Nonspecific Stimulation of Bovine T and B Cells by Lymphostatin from Attaching and Effacing Escherichia coli.
Infect Immun. 85 (2): e00845-16. -
Jimbo, S. et al. (2019) Natural and inducible regulatory B cells are widely distributed in ovine lymphoid tissues.
Vet Immunol Immunopathol. 211: 44-8. -
Stabel, J.R. & Bannantine, J.P. (2019) Divergent Antigen-Specific Cellular Immune Responses during Asymptomatic Subclinical and Clinical States of Disease in Cows Naturally Infected with Mycobacterium avium. subsp. paratuberculosis.
Infect Immun. 88(1):e00650-19. -
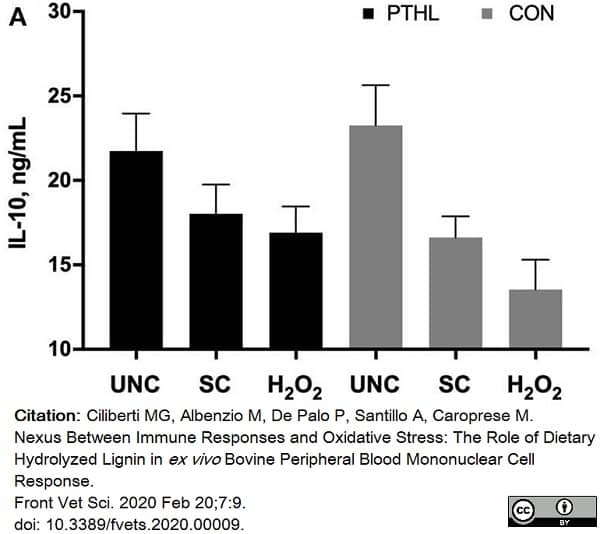
Ciliberti, M.G. et al. (2020) Nexus Between Immune Responses and Oxidative Stress: The Role of Dietary Hydrolyzed Lignin in ex vivo Bovine Peripheral Blood Mononuclear Cell Response.
Front Vet Sci. 7: 9. -
Stabel, J.R. et al. (2021) Comparative cellular immune responses in calves after infection with Mycobacterium avium. subsp. paratuberculosis., M. avium. subsp. avium., M. kansasii. and M. bovis..
Vet Immunol Immunopathol. 237: 110268. -
Davidson, J.O. et al. (2021) Window of opportunity for human amnion epithelial stem cells to attenuate astrogliosis after umbilical cord occlusion in preterm fetal sheep.
Stem Cells Transl Med. 10 (3): 427-40. -
Ciliberti, M.G. et al. (2022) Green extraction of bioactive compounds from wine lees and their bio-responses on immune modulation using in vitro sheep model.
J Dairy Sci. 105 (5): 4335-53. -
Santillo, A. et al. (2022) Feeding tannins to dairy cows in different seasons improves the oxidative status of blood plasma and the antioxidant capacity of cheese.
J Dairy Sci. 105 (11): 8609-20. -
Bouroutzika, E. et al. (2023) Melatonin Administration to Pregnant Ewes for Coccidiosis Control in Their Offspring.
Animals (Basel). 13 (14): 2381. -
de Silva. K. et al. (2018) Defining resilience to mycobacterial disease: Characteristics of survivors of ovine paratuberculosis.
Vet Immunol Immunopathol. 195: 56-64. -
Stabel, J.R. et al. (2020) Comparison of Sheep, Goats, and Calves as Infection Models for Mycobacterium avium subsp. paratuberculosis.
Vet Immunol Immunopathol. 225: 110060. -
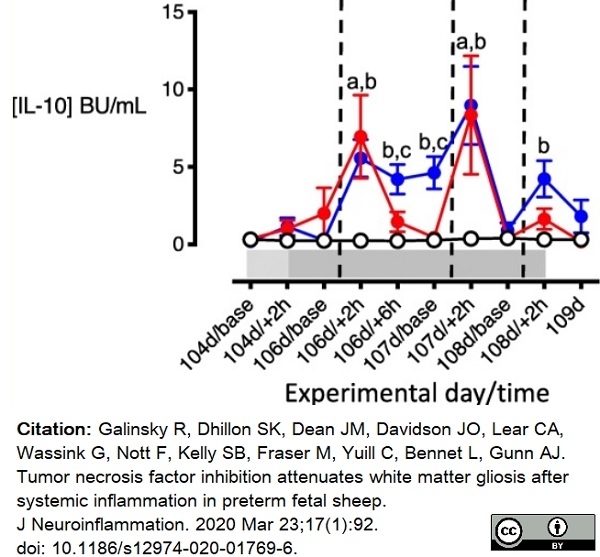
Galinsky, R. et al. (2020) Tumor necrosis factor inhibition attenuates white matter gliosis after systemic inflammation in preterm fetal sheep.
J Neuroinflammation. 17 (1): 92. -
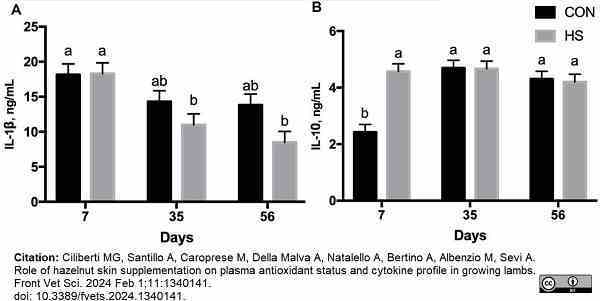
Ciliberti, M.G. et al. (2024) Role of hazelnut skin supplementation on plasma antioxidant status and cytokine profile in growing lambs.
Front Vet Sci. 11: 1340141.
- RRID
- AB_2249021
- UniProt
- P43480
- Entrez Gene
- IL10
- GO Terms
- GO:0002237 response to molecule of bacterial origin
- GO:0002740 negative regulation of cytokine secretion involved in immune response
- GO:0002904 positive regulation of B cell apoptosis
- GO:0005125 cytokine activity
- GO:0005615 extracellular space
- GO:0006954 inflammatory response
- GO:0006955 immune response
- GO:0030889 negative regulation of B cell proliferation
- GO:0032715 negative regulation of interleukin-6 production
- View More GO Terms
- GO:0032800 receptor biosynthetic process
- GO:0042095 interferon-gamma biosynthetic process
- GO:0043193 positive regulation of gene-specific transcription
- GO:0045019 negative regulation of nitric oxide biosynthetic process
- GO:0045077 negative regulation of interferon-gamma biosynthetic process
- GO:0050715 positive regulation of cytokine secretion
- GO:0051045 negative regulation of membrane protein ectodomain proteolysis
- GO:0051091 positive regulation of transcription factor activity
- GO:0051384 response to glucocorticoid stimulus
MCA2110
If you cannot find the batch/lot you are looking for please contact our technical support team for assistance.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Bovine ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up