Porcine Adaptive Immunity
Pig Adaptive Immune System Cells
The adaptive immune system is essential for the host’s response to bacteria, pathogenic viruses and vaccination. The adaptive response is coordinated by B cells that produce antibodies and T cells that kill virus infected cells and secrete cytokines that regulate the immune system. Unlike the innate immune response, the adaptive immune response takes longer to respond to a specific antigen, but on repeat exposures it responds rapidly due to memory B and T cells.
T Cells
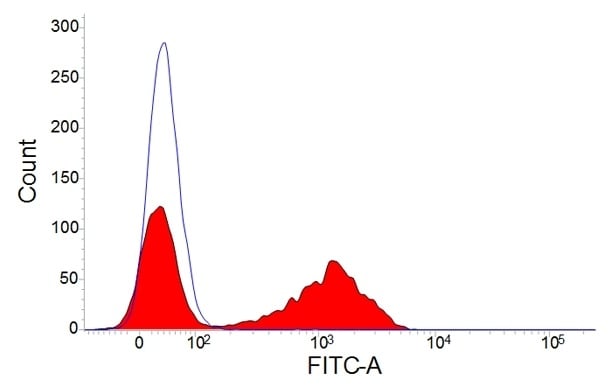
Staining of porcine peripheral blood lymphocytes with mouse anti-pig CD3:FITC (MCA5951F).
In addition to single positive peripheral blood CD3+CD4+ TCRalphabeta+ and CD3+CD8+ TCRalphabeta+ T cells, the pig also has double positive and double negative TCRalphabeta+ peripheral T cell populations as well as gammadelta T cells. The unique double positive T cell subset (CD4+CD8alpha+TCRalphabeta+) is usually only found in the thymus in humans and mice. The majority of the double positive population has low levels of CD3 (Sinkora M et al. 2000).
There are higher levels of gammadelta T cells in pigs than in humans or mice and the majority do not express CD4 or CD8 (CD3+CD8alpha-TCRgammadelta+ and CD3+CD4-TCRgammadelta+), but some express CD8alpha (CD3+CD8alpha+TCR-gammadelta+). All pig CD4+ T cells co-express the CD2, CD3 and CD5 antigens.
CD4+ T cells are further classified into two T helper cell groups (Th1 and Th2) based on their cytokine production profiles. IFN-gamma producing Th1 cells activate macrophages, whereas interleukin-4 (IL-4) secreting Th2 cells stimulate B cells.
Peripheral double positive (CD3+CD4+CD8alpha+TCRalphabeta+) T cells recall antigen and their numbers increase as the pig ages. It is thought that these cells may be activated and/or memory T-helper cells.
Pig T-helper cells can be functionally divided into subpopulations by their expression levels of CD27 (Reutner et al. 2013). All naïve CD8alpha- T cells express CD27 and reside in various lymph nodes. In contrast, not all CD4+CD8alpha+ T helper cells express CD27: CD8alpha+CD27+ and CD8alpha+CD27- cells are found in spleen, liver and blood and produce IFN-gamma upon stimulation. Of these two CD8alpha+ populations, the CD8alpha+CD27- cells seem to be terminally differentiated effector memory cells as indicated by their slower proliferation rate and higher production of IFN-gamma and TNF-alpha.
All CD4-CD8+ T cells co-express CD2, but can be subdivided based on the presence or absence of co-expression of CD3, CD5 and CD6. So within the cytotoxic CD4-CD8+ T cell population two subsets can be defined by the presence or absence of the CD6 differentiation antigen. CD6- cells demonstrate spontaneous non-MHC restricted cytolytic activity, whereas CD6+ cells are MHC Class-I restricted cytolytic CD4-CD8+ T cells (Saalmüller et al. 1999).
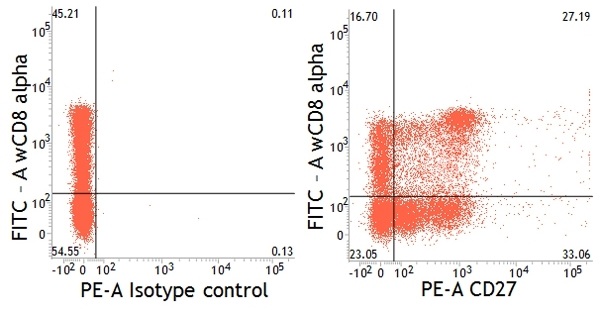
Dual staining of pig peripheral blood lymphocytes with mouse anti-pig CD27:RPE (MCA5973PE) and mouse anti-pig wCD8a:FITC (MCA1223F).
Gammadelta T Cells
Pig gammadelta T cells also differ from humans and mice in that they are more abundant in the peripheral blood. It is thought that they are important in responding to non-peptide antigens, antigen presentation, cytolytic activity, and memory function. Although most gammadelta T cells do not express either CD4 or CD8, a few express CD8 (CD3+CD8alpha+TCR gammadelta-).
Unlike humans and mice, pig gammadelta T cells can be separated into two subpopulations based on the absence or presence of CD2: CD4-CD8-CD2- gammadelta TCR+ and CD4-CD8-CD2+ gammadelta TCR+. It is thought that they can carry out functions similar to antigen presenting cells and therefore are able to present antigen to CD4+ T cells by direct interaction via MHC class II (Takamatsu et al. 2006).
B Cells
The B cell receptor (BCR) complex is comprised of heavy and light chain immunoglobulin molecules and the heterodimers CD79alpha and CD79beta. Pig B cells, but not T cells, express CD79beta making it a useful B cell marker. Pig CD79alpha has been cloned and RT-PCR revealed that Cd79alpha mRNA is mainly detected in lymphoid tissues being highest in the spleen and mesenteric lymph nodes and lowest in small intestines, lung and thymus. Although there isn’t a specific monoclonal antibody against pig CD79alpha, an anti-human CD79alpha monoclonal antibody recognizes pig CD79alpha. Immunohistochemistry staining of the spleen shows that CD79alpha is strongly expressed in the follicular mantle zone, rather than the germinal center (Lee et al. 2008).
As in humans and mice, pig B cell lymphogenesis occurs in the bone marrow (BM). There is no pan B cell CD marker for swine. However, pig B cells can be detected using anti-µHC (IgM heavy chain) which omits B cell precursors and most plasma cells. In the BM sequential stages of B cell development can be identified by seven phenotypic subsets (Sinkora and Sinkorova 2014) using MHC Class II, CD2, CD21, CD25, CD45RC, CD172a, SWC7 (swine workshop cluster 7) and µHC (IgM heavy chain):
| Phenotypes and precursor stage of key subsets during B cell development | |
|---|---|
| CD72abriSWC7-CD45RC-CD2-CD25-CD21a-CD21b-mHC- | Large common precursor |
| CD72ahiSWC7-CD45RC-/loCD2-/+CD25-/loCD21a-CD21b-mHC- | Large proB cell |
| CD72aloSWC7hiCD45RChiCD2-/+CD25-/loCD21a-/loCD21b-mHC- | Large and small preB-1 populations |
| CD72a-SWC7-/loCD45RC-/lo/hiCD2+CD25hiCD21ahiCD21b-mHClo | Large and small preB-II populations |
| CD72a-SWC7-/loCD45RCbriCD2+CD25hiCD21abriCD21b+mHChi | Small immature and small naïve B cell populations |
CD21 and CD2 are useful B cell differentiation markers in the pig. Peripheral pig IgM+ mature B cells can be divided into four different populations depending on their expression of CD2 and CD21b: CD2+CD21+ (mainly naïve B cells), CD2-CD21+ (primed B cells), CD2+CD21- (active antibody forming and plasma cells) and CD2-CD21- (resting antibody forming and plasma cells). During porcine reproductive and respiratory syndrome virus (PRRSV) infection, B cells rapidly differentiate; as the number of antibody forming cells (CD2+CD21-) increases the number of CD2+CD21- primed B cells (CD2-CD21+) drastically drops (Sinkora et al. 2009; Sinkora et al. 2014).
Although peripheral B cells are generally negative for CD172a, some mature B cells can be induced to express low levels of CD172a after antigen stimulation.
CD5 is usually expressed on T cells, but it can also be found on a small population of B cells. CD5 expression in humans and mice identifies two subsets of B cells: B1 (CD5+) and B2 (CD5-) B cells. The B1+ cells are potent producers of IL-10. Unlike humans and mice, pig B1 and B2 cells cannot be differentiated into two populations based on their expression of the CD5.
Our pig antibodies range will further help you to investigate the adaptive side of the porcine immune system.
References
- Lee S et al. (2008). Molecular cloning and expression analysis of pig CD79alpha. Vet. Immunol. Immunopathol. 125(3-4):368
- Reutner K et al. (2013). CD27 expression discriminates porcine T helper cells with functionally distinct properties. Vet. Res. 44(1):18
- Saalmüller A et al. (1999). Characterization of porcine T lymphocytes and their immune response against viral antigens. J. Biotechnol. 73(2-3):223
- Sinkora M et al. (2000). Early Ontogeny of Thymocytes in Pigs: Sequential Colonization of the Thymus by T Cell Progenitors. J. Immunol. 165(4):1832
- Sinkora M and Butler J. (2009). The ontogeny of the porcine immune system. Dev. Comp. Immunol. 33(3):273
- Sinkora M and Sinkorova J (2014). B cell lymphogenesis in swine is located in the bone marrow. J. Immunol. 193(10):5023
- Sinkora M et al. (2014). The comparative profile of lymphoid cells and the T and B cell spectratype of germ-free piglets infected with viruses SIV, PRRSV or PCV2. Vet Res. 45(1):91
- Takamatsu H et al. (2006). Porcine gammadelta T cells: possible roles on the innate and adaptive immune responses following virus infection. Vet. Immunol. Immunopathol. 112(1-2):49



