Porcine CD27 - a Marker for Porcine Immune Cell Subsets
Overview
The pig immunology system is not only important to understand porcine immunity for improving animal welfare in farming, but also has relevance to translational medicine as a large model animal in human medicine (Meurens et al. 2012, Bailey et al. 2013). One of the factors that has delayed this research is a lack of porcine specific research reagents. While antibody availability is not at the level of the rodent animal models, improvements are ongoing; among these is the anti-porcine CD27 antibody, clone b30c7 (Reutner et al. 2012, Reutner et al. 2013), which has enabled further insights into porcine T helper (Th), natural killer (NK) cells, and intraepithelial T cells (IET).
CD27
CD27 belongs to the tumor necrosis factor receptor (TNFR) superfamily. It is a glycosylated, 55 kDa type I transmembrane protein, expressed as a disulfide linked homodimer on the cell surface. It is found on the surface of B, T, and NK cell subsets. CD70 is the ligand for CD27 and upon binding generates costimulatory signals leading to activation and differentiation of T cells into effector and memory cells, and it also regulates B cell activation (Prasad et al. 1997). It is being investigated for a possible role in immunomodulation for cancer therapy (Marin-Acevedo et al. 2018).
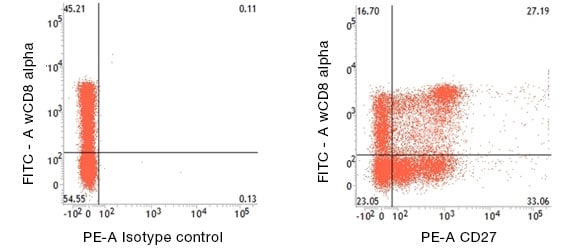
Fig.1. CD27 expression on porcine T helper cells in pig peripheral blood lymphocytes detected with Mouse Anti-Pig CD27:RPE (MCA5973PE) and Mouse Anti-Pig wCD8a:FITC (MCA1223F).
Porcine CD27 - T Helper Cells
An unusual aspect of porcine Th cell biology is the expression of CD8α on a large subset of these CD4+ cells in the peripheral immune system (Saalmüller et al. 1987). Generation of the CD27 antibody provided much additional information about the differentiation of Th cells (Reutner et al. 2012, Reutner et al. 2013) (Figure 1). Specifically, all naïve CD8α- Th cells express CD27, but the CD8α+ Th cells split into a CD27+ and a CD27- subset. Their organ distribution is as follows:
- Naïve CD8α-CD27+ Th cells mainly reside in lymph nodes
- CD8α+CD27+ and CD8α+CD27- Th cells can be found in blood, spleen, and liver
CD4+CD8α+CD27- Th cells generally have no CCR7 and very low levels of CD62L mRNA, and generate the least proliferative response to antigenic stimulation. However, they are better at IFN-γ and TNF-α production. Together, these factors suggest a functional equivalence to human effector memory T cells. Similarly, CD4+CD8α+CD27+ Th cells, have increased expression of CCR7 and CD62L and secrete intermediate levels of IFN-γ and TNF-α, demonstrating the characteristics of central memory cells.
Porcine CD27 ─ Natural Killer Cells
In humans, NK cells can be subdivided based on the combined expression levels of CD56 with CD16, and of CD11b and CD27 (Cooper et al. 2001, Fu et al. 2011). The latter two receptors also apply to mouse NK cell subtypes (Hayakawa and Smyth 2006).
The availability of antibodies to porcine CD27 and NKp46 (CD335) has made it possible to extend this analysis to pigs (Mair et al. 2012). Pigs also have CD335- and CD335+ cells, exhibiting NK phenotypes, in the circulation. Additionally, in the spleen, a third NK phenotype cell can be found that has low to negative CD8α and increased CD335 levels. This population has higher levels of porcine CD16 and CD27, relative to the spleen and blood CD8α+ CD335- and CD8α+ CD335+ NK cell subsets (Mair et al. 2013).
Porcine CD27 ─ Intraepithelial T Cells
Intraepithelial T cells (IETs), as the name indicates, are the resident T cells of the intestinal tract epithelial layer and their functions are varied:
- Respond to foreign antigen from the intestinal lumen, such as enteric pathogens
- Regulate metabolism
- Secrete cytokines and trigger intestinal inflammation
- Are subject to regulation but also carry out regulatory functions
(Montufar-Solis et al. 2007, Mowat and Agace 2014, He et al. 2019).
In pigs, the majority of IETs are CD2+CD8α+ γδ and CD4−CD8α+ αβ T cells. However, within this, there is variation in the distribution of IETs when examining pigs in the critical 4- to 8-week nursery age range (Wiarda et al. 2020):
- CD2+CD8α− γδ IETs predominate in the small intestine
- CD2+CD8α+ γδ IETs are at higher levels in the large intestine
- CD4−CD8α+ αβ IETs increase progressively over time in all sites
- In older pigs the large intestine has higher levels of γδ IETs than the small intestine
The percentages of CD27 expressing T cells in the primary IETs (CD2+CD8α+ γδ IETs and CD4−CD8α+ αβ) decrease in the ileum and large intestine with age. Toward the end of the nursery phase, the large intestine also has fewer CD27+ IETs compared to the small intestine. The implication being that the 4- to 8-weeks of age is a key developmental phase of IET maturation (Wiarda et al. 2020).
Porcine Antibodies
Bio-Rad is the leading supplier of directly reactive antibodies to veterinary and translational animal models. Select from our porcine CD marker antibody range for immune cell analysis and complement it with cytokine and chemokine ELISA pairs for immune function analysis. Finally, the key antibody in this micro-review, Porcine CD27 (MCA5973), is available from Bio-Rad in APC, FITC, RPE, and purified formats; ideal for multicolor flow cytometry.
References
- Bailey M et al. (2013). The evolutionary basis for differences between the immune systems of man, mouse, pig and ruminants. Vet Immunol Immunopathol. 152(1-2), 13-19.
- Cooper MA et al. (2001). Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 97(10), 3146-3151.
- Fu B et al. (2011). CD11b and CD27 reflect distinct population and functional specialization in human natural killer cells. Immunol. 133(3), 350-359.
- Hayakawa Y and Smyth MJ (2006). CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 176(3), 1517-1524.
- He S et al. (2019). Gut intraepithelial T cells calibrate metabolism and accelerate cardiovascular disease. Nature. 566(7742), 115-119.
- Mair KH et al. (2012). NKp46 expression discriminates porcine NK cells with different functional properties. Eur J Immunol. 42(5), 1261-1271.
- Mair KH et al. (2013). Porcine CD8αdim/-NKp46high NK cells are in a highly activated state. Vet Res. 44(1), 13.
- Marin-Acevedo JA et al. (2018). Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol. 11(1), 39.
- Meurens F et al. (2012). The pig: a model for human infectious diseases. Trends Microbiol. 20(1), 50-57.
- Montufar-Solis D et al. (2007). T-cell activation in the intestinal mucosa. Immunol Rev. 215, 189-201.
- Mowat AM and Agace WW (2014). Regional specialization within the intestinal immune system. Nature reviews. Immunol. 14(10), 667-685.
- Prasad KV et al. (1997). CD27, a member of the tumor necrosis factor receptor family, induces apoptosis and binds to Siva, a proapoptotic protein. Proc Natl Acad Sci U S A. 94(12), 6346-6351.
- Reutner K et al. (2012). Porcine CD27: identification, expression and functional aspects in lymphocyte subsets in swine. Dev Comp Immunol. 38, 321-31.
- Reutner K et al. (2013). CD27 expression discriminates porcine T helper cells with functionally distinct properties. Vet Res. 44, 18.
- Saalmüller A et al. (1987). Simultaneous expression of CD4 and CD8 antigens by a substantial proportion of resting porcine T lymphocytes. Eur J Immunol. 17(9), 1297-1301.
- Wiarda JE et al. (2020). Intraepithelial T cells diverge by intestinal location as pigs age. Front Immunol. 11, 1139.



