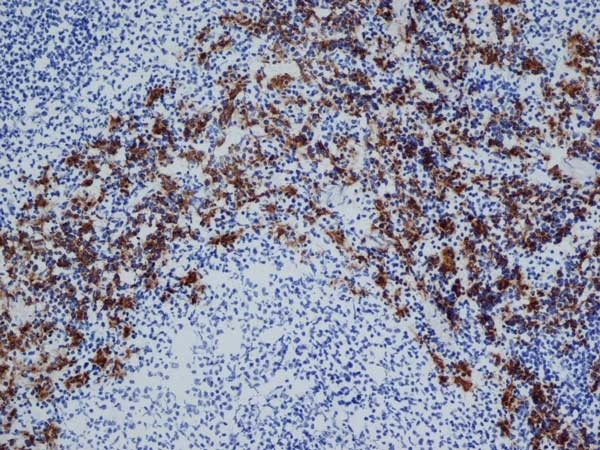
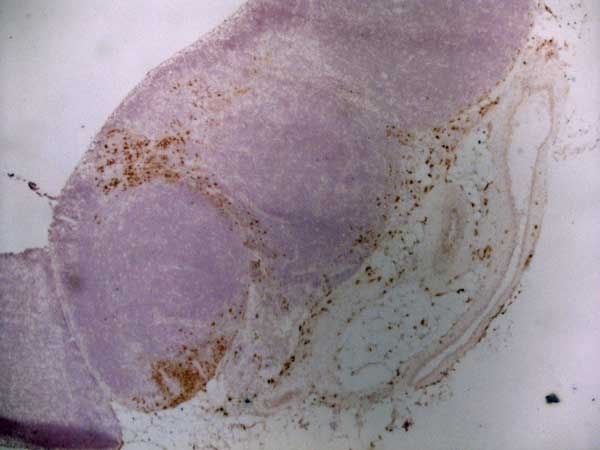
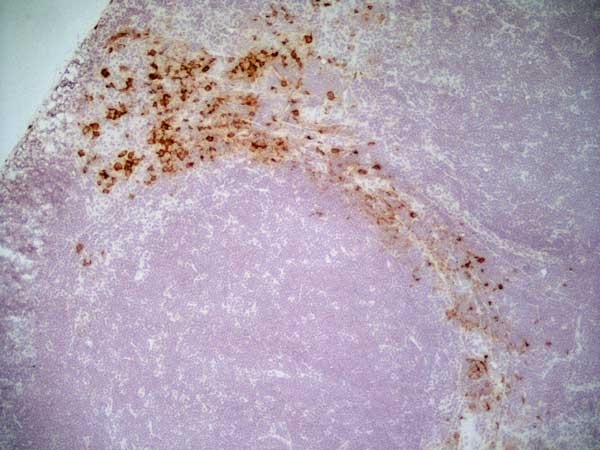
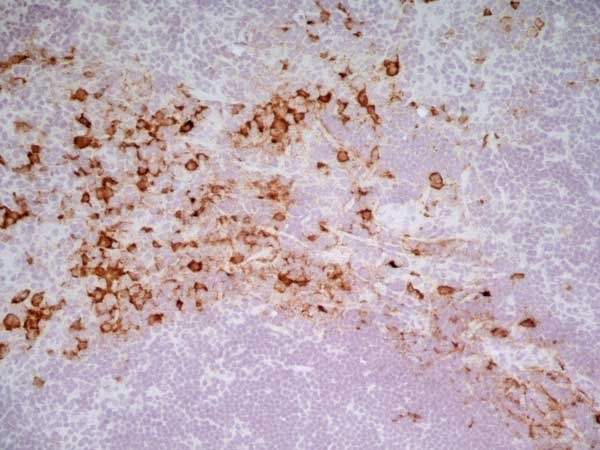
CD163 antibody | ED2














Mouse anti Rat CD163
- Product Type
- Monoclonal Antibody
- Clone
- ED2
- Isotype
- IgG1
- Specificity
- CD163
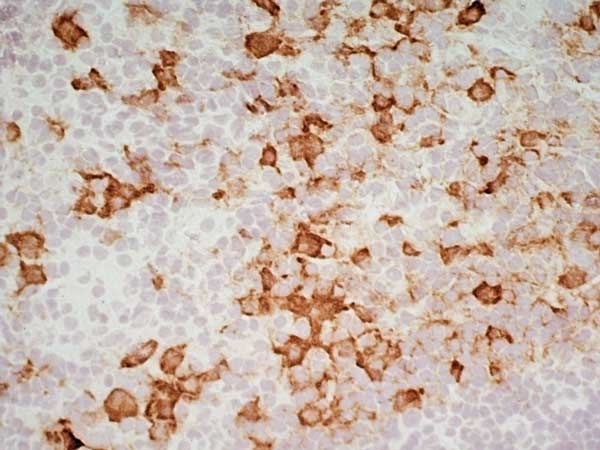
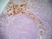
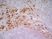
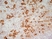
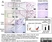
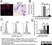
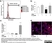
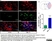
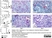
| Mouse anti Rat CD163, clone ED2 recognizes the rat ED2 cell surface glycoprotein (Dijkstra et al. 1985). A 175 kDa molecule also known as rat CD163, a member of the group B scavenger receptor cysteine-rich (SRCR) family and an erythroblast adhesion receptor (Fabriek et al. 2007). Mouse anti rat CD163, clone ED2 was shown to detect approximately 50% of peritoneal macrophages, a subset of splenic macrophages, and most tissue macrophages. However, no staining was observed in monocytes or alveolar macrophages (Dijkstra et al. 1985, Beelen et al. 1987). In freshly isolated bone marrow, expression of CD163 was limited to mature macrophages only (Barbe et al. 1990). Clone ED2 may be used in immunohistology using antigen retrieval, and has also been described reacting with paraffin-embedded material following PLP fixation (Periodate-lysine-paraformaldehyde), see Whiteland et al. |

Our CD163 (ED2) Antibody has been referenced in >116 publications* *Based on June 2020 data from CiteAb's antibody search engine. |
- Target Species
- Rat
- Product Form
- Purified IgG - liquid
- Preparation
- Purified IgG prepared by affinity chromatography on Protein A from tissue culture supernatant
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
0.09% Sodium Azide - Carrier Free
- Yes
- Immunogen
- Rat spleen cell homogenate.
- Approx. Protein Concentrations
- IgG concentration 0.5 mg/ml
- Fusion Partners
- Spleen cells from immunized BALB/c mice were fused with cells of the SP2/0-Ag 14 mouse myeloma cell line.
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Flow Cytometry | |||
| Immunofluorescence | |||
| Immunohistology - Frozen | 1/50 | 1/100 | |
| Immunohistology - Paraffin 1 | |||
| Immunoprecipitation | |||
| Western Blotting |
- 1 This product requires protein digestion pre-treatment of paraffin sections e.g. trypsin or pronase
- Flow Cytometry
- Use 10ul of the suggested working dilution to label 1x106 cells in 100ul
- Histology Positive Control Tissue
- Liver
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Mouse IgG1 Negative Control | MCA1209 | F | 0.1 mg |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Mouse IgG1 Negative Control | ||||||
Source Reference
-
Dijkstra, C.D. et al. (1985) The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3.
Immunology. 54 (3): 589-99.
References for CD163 antibody
-
Barbe, E. et al. (1990) Characterization and expression of the antigen present on resident rat macrophages recognized by monoclonal antibody ED2.
Immunobiol. 182: 88-99. -
Dijkstra, C.D. & Damoiseaux, J.G. (1993) Macrophage heterogeneity established by immunocytochemistry.
Prog Histochem Cytochem. 27 (2): 1-65. -
Whiteland, J.L. et al. (1995) Immunohistochemical detection of T-cell subsets and other leukocytes in paraffin-embedded rat and mouse tissues with monoclonal antibodies.
J Histochem Cytochem. 43 (3): 313-20. -
Muller, D.N. et al. (2002) Immunosuppressive treatment protects against angiotensin II-induced renal damage.
Am J Pathol. 161: 1679-93. -
Polfliet, M.M.J. et al. (2002) Identification of the rat mature macrophage antigen ED2 as CD163: Regulation by glucocorticoids and role in the production of proinflammatory mediators.
PhD Thesis. Vrije University, Amsterdam. -
Banerjee, S. et al. (2003) Development of organised conjunctival leucocyte aggregates after corneal transplantation in rats.
Br J Ophthalmol. 87: 1515-22. -
Moghaddami, M. et al. (2005) MHC class II compartment, endocytosis and phagocytic activity of macrophages and putative dendritic cells isolated from normal tissues rich in synovium.
Int Immunol. 17: 1117-30. -
Ghiringhelli, F. et al. (2005) Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation.
J Exp Med. 202: 919-29.
View The Latest Product References
-
Deng, X. et al. (2005) Chronic alcohol consumption accelerates fibrosis in response to cerulein-induced pancreatitis in rats.
Am J Pathol. 166 (1): 93-106. -
Fabriek, B.O. et al. (2007) The macrophage CD163 surface glycoprotein is an erythroblast adhesion receptor.
Blood. 109 (12): 5223-9. -
Wehner, S. et al. (2007) Inhibition of macrophage function prevents intestinal inflammation and postoperative ileus in rodents.
Gut. 56: 176-85. -
Hamada, T. et al. (2007) Oncostatin M gene therapy attenuates liver damage induced by dimethylnitrosamine in rats.
Am J Pathol. 171: 872-81. -
Keitel, V. et al. (2008) Expression and function of the bile acid receptor TGR5 in Kupffer cells.
Biochem Biophys Res Commun. 372: 78-84. -
Starke-Buzetti, W.A. et al. (2008) Increased glial-derived neurotrophic factor in the small intestine of rats infected with the tapeworm, Hymenolepis diminuta.
Int J Exp Pathol. 89: 458-65. -
Jensen, K.T. et al. (2008) Early inflammatory response of knee ligaments to prolotherapy in a rat model.
J Orthop Res. 26: 816-23. -
Rosas-Ballina, M. et al. (2008) Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia.
Proc Natl Acad Sci U S A. 105: 11008-13. -
Santos, M. et al. (2009) An unbiased stereological study on subpopulations of rat liver macrophages and on their numerical relation with the hepatocytes and stellate cells.
J Anat. 214: 744-51. -
Yamato, M. et al. (2009) PET and macro- and microautoradiographic studies combined with immunohistochemistry for monitoring rat intestinal ulceration and healing processes.
J Nucl Med. 50: 266-73. -
Yu, Y. et al. (2010) Brain perivascular macrophages and the sympathetic response to inflammation in rats after myocardial infarction.
Hypertension. 55: 652-9. -
Loureiro, J. et al. (2010) BMP-7 blocks mesenchymal conversion of mesothelial cells and prevents peritoneal damage induced by dialysis fluid exposure.
Nephrol Dial Transplant. 25: 1098-108. -
Bedi, A. et al. (2010) Effect of early and delayed mechanical loading on tendon-to-bone healing after anterior cruciate ligament reconstruction.
J Bone Joint Surg Am. 92: 2387-401. -
Harty, M.W. et al. (2010) Neutrophil depletion blocks early collagen degradation in repairing cholestatic rat livers.
Am J Pathol. 176: 1271-81. -
Fujita, E. et al. (2010) Statin attenuates experimental anti-glomerular basement membrane glomerulonephritis together with the augmentation of alternatively activated macrophages.
Am J Pathol. 177 (3): 1143-54. -
Zeng, Z. et al. (2010) Heme oxygenase-1 protects donor livers from ischemia/reperfusion injury: the role of Kupffer cells.
World J Gastroenterol. 16: 1285-92. -
Dessem, D. et al. (2010) Eccentric muscle contraction and stretching evoke mechanical hyperalgesia and modulate CGRP and P2X(3) expression in a functionally relevant manner.
Pain. 149: 284-95. -
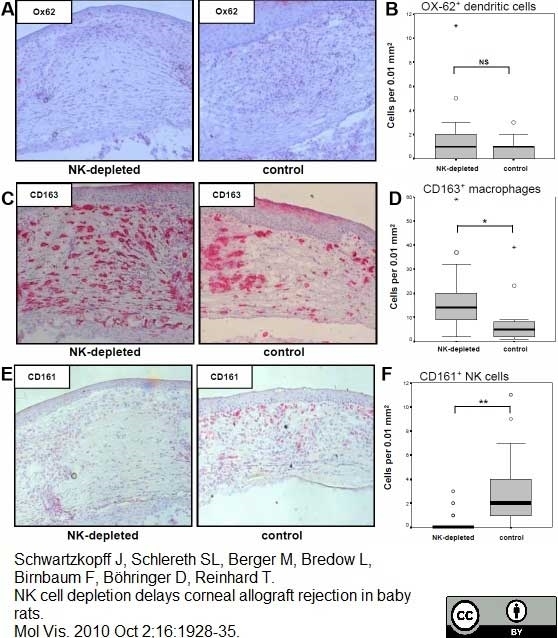
Schwartzkopff, J. et al. (2010) NK cell depletion delays corneal allograft rejection in baby rats.
Mol Vis. 16: 1928-35. -
Teng, B.T. et al. (2011) Protective effect of caspase inhibition on compression-induced muscle damage.
J Physiol. 589: 3349-69. -
Kajita, M. et al. (2011) iNOS expression in vascular resident macrophages contributes to circulatory dysfunction of splanchnic vascular smooth muscle contractions in portal hypertensive rats.
Am J Physiol Heart Circ Physiol. 300: H1021-31. -
Baker, S.C. et al. (2011) Cellular integration and vascularisation promoted by a resorbable, particulate-leached, cross-linked poly(ε-caprolactone) scaffold.
Macromol Biosci. 11 (5): 618-27. -
Kawakami, A.P. et al. (2011) Inflammatory Process Modulation by Homeopathic Arnica montana 6CH: The Role of Individual Variation.
Evid Based Complement Alternat Med. 2011: 917541. -
Wojcik, M. et al. (2012) Immunodetection of cyclooxygenase-2 (COX-2) is restricted to tissue macrophages in normal rat liver and to recruited mononuclear phagocytes in liver injury and cholangiocarcinoma.
Histochem Cell Biol. 137: 217-33. -
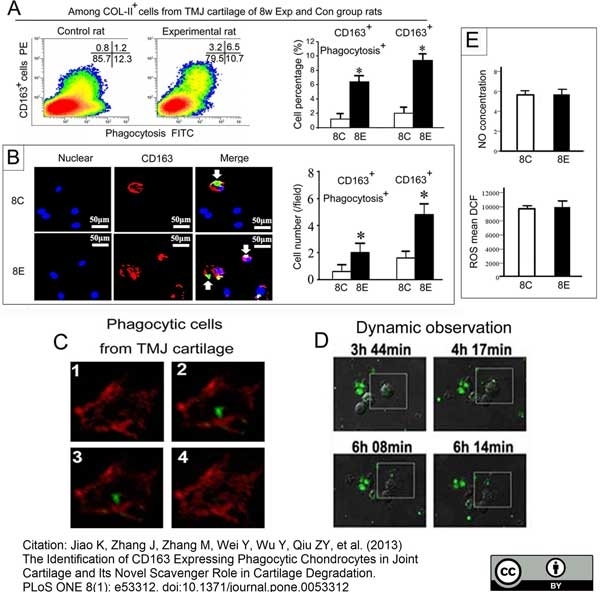
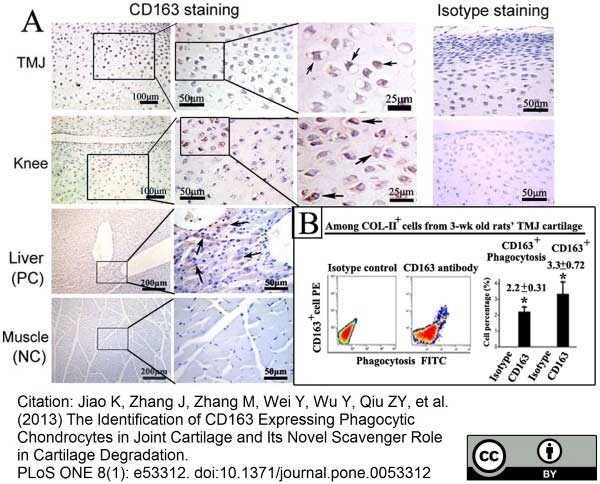
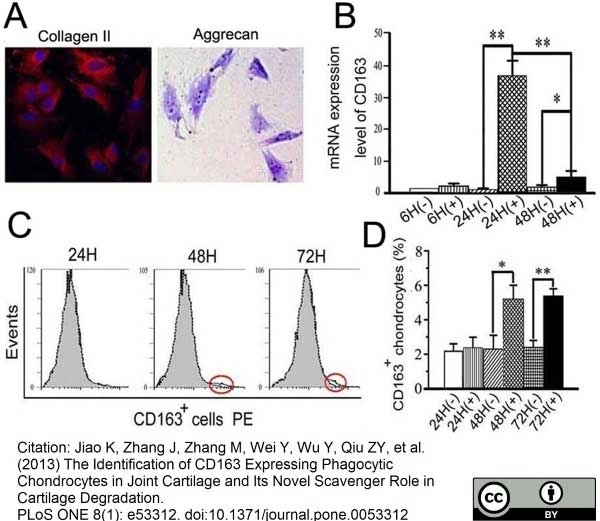
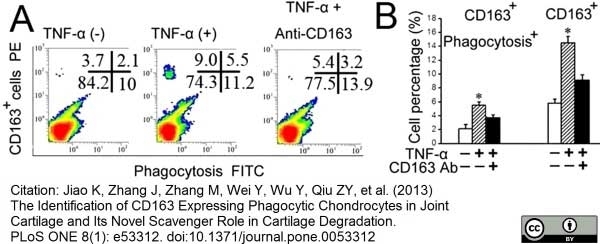
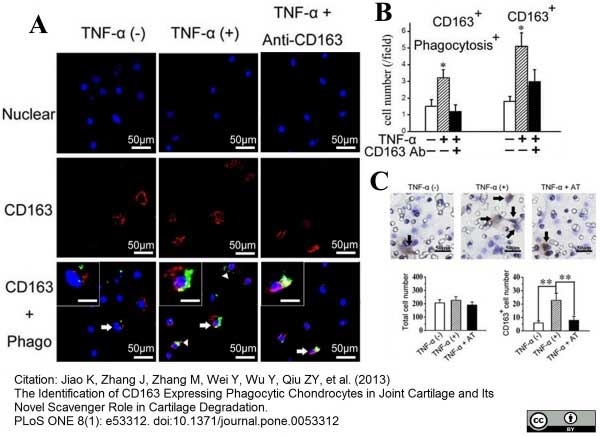
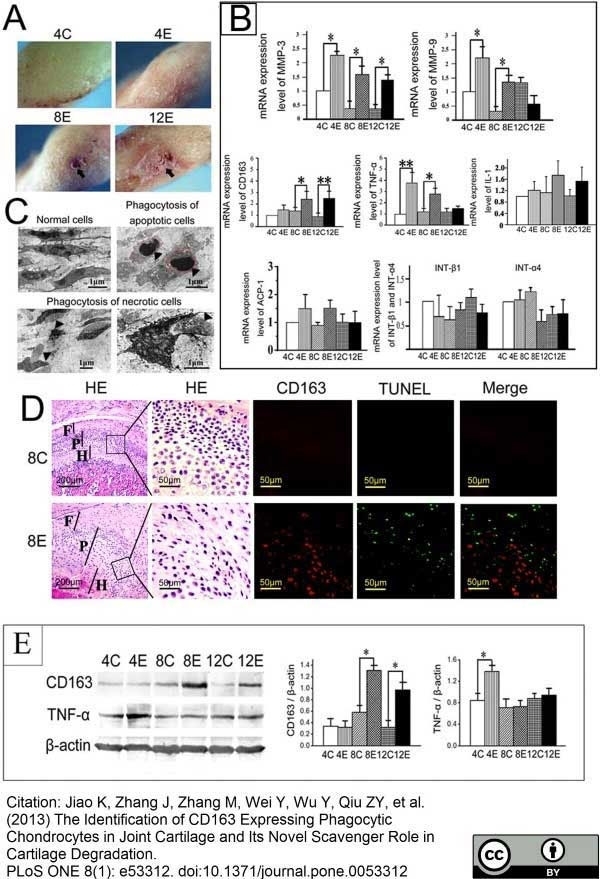
Jiao, K. et al. (2013) The Identification of CD163 Expressing Phagocytic Chondrocytes in Joint Cartilage and Its Novel Scavenger Role in Cartilage Degradation.
PLoS One. 8(1):e53312. -
Duchesne, E. et al. (2013) Mast cells can regulate skeletal muscle cell proliferation by multiple mechanisms.
Muscle Nerve. 48 (3): 403-14. -
Fujii, Y. et al. (2013) Effect of enzymatically modified isoquercitrin on preneoplastic liver cell lesions induced by thioacetamide promotion in a two-stage hepatocarcinogenesis model using rats.
Toxicology. 305: 30-40. -
Lobato-Pascual, A. et al. (2013) Rat macrophage C-type lectin is an activating receptor expressed by phagocytic cells.
PLoS One. 8: e57406. -
Park, E.S. et al. (2014) Establishment of a rat model for canine necrotizing meningoencephalitis (NME).
Vet Pathol. 51 (6): 1151-64. -
Han, T.T. et al. (2015) Adipose-derived stromal cells mediate in vivo adipogenesis, angiogenesis and inflammation in decellularized adipose tissue bioscaffolds.
Biomaterials. 72: 125-37. -
Stavenuiter, A.W. et al. (2015) Protective Effects of Paricalcitol on Peritoneal Remodeling during Peritoneal Dialysis.
Biomed Res Int. 2015: 468574. -
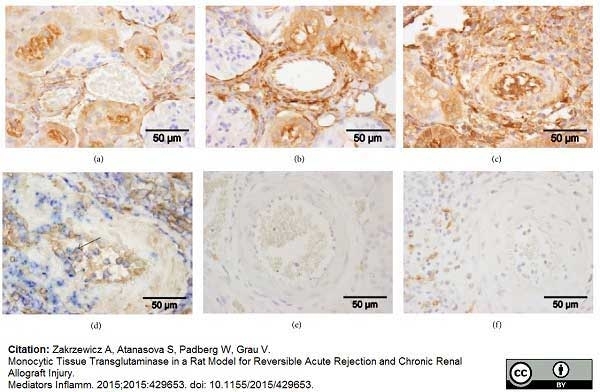
Zakrzewicz, A. et al. (2015) Monocytic Tissue Transglutaminase in a Rat Model for Reversible Acute Rejection and Chronic Renal Allograft Injury.
Mediators Inflamm. 2015: 429653. -
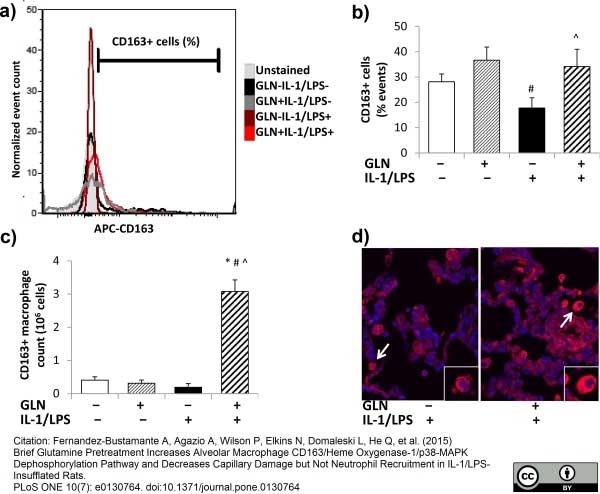
Fernandez-Bustamante, A. et al. (2015) Brief Glutamine Pretreatment Increases Alveolar Macrophage CD163/Heme Oxygenase-1/p38-MAPK Dephosphorylation Pathway and Decreases Capillary Damage but Not Neutrophil Recruitment in IL-1/LPS-Insufflated Rats.
PLoS One. 10 (7): e0130764. -
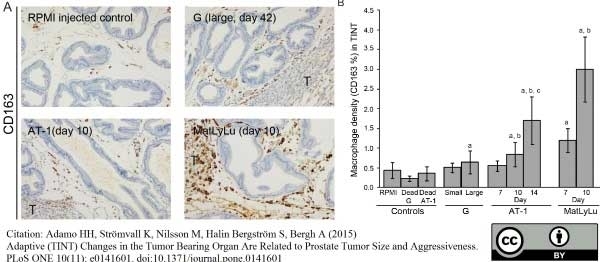
Adamo, H.H. et al. (2015) Adaptive (TINT) Changes in the Tumor Bearing Organ Are Related to Prostate Tumor Size and Aggressiveness.
PLoS One. 10 (11): e0141601. -
Tentillier N et al. (2016) Anti-Inflammatory Modulation of Microglia via CD163-Targeted Glucocorticoids Protects Dopaminergic Neurons in the 6-OHDA Parkinson's Disease Model.
J Neurosci. 36 (36): 9375-90. -
Pannell, M. et al. (2016) Adoptive transfer of M2 macrophages reduces neuropathic pain via opioid peptides.
J Neuroinflammation. 13 (1): 262. -
Almahrog, A.J. et al. (2016) In vivo. association of immunophenotyped macrophages expressing CD163 with PDGF-β in gingival overgrowth-induced by three different categories of medications.
J Oral Biol Craniofac Res. 6 (1): 10-7. -
Ibarra, V. et al. (2016) Evaluation of the tissue response to alginate encapsulated islets in an omentum pouch model.
J Biomed Mater Res A. 104 (7): 1581-90. -
Wang, M. et al. (2017) Characterization of the Micro-Environment of the Testis that Shapes the Phenotype and Function of Testicular Macrophages.
J Immunol. 198 (11): 4327-40. -
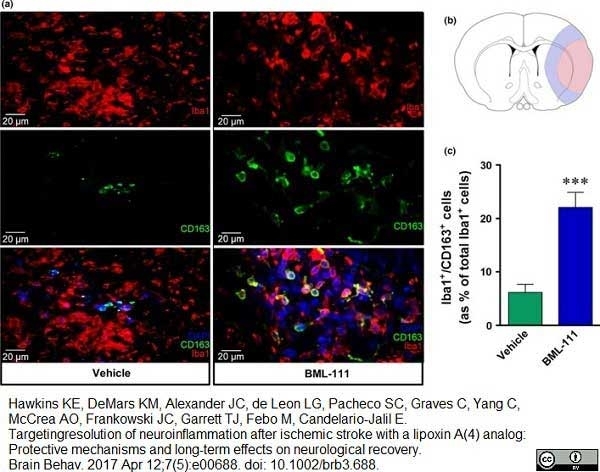
Hawkins, K.E. et al. (2017) Targeting resolution of neuroinflammation after ischemic stroke with a lipoxin A4 analog: Protective mechanisms and long-term effects on neurological recovery.
Brain Behav. 7 (5): e00688. -
Chang, J.C. et al. (2019) Early Immune Response to Acute Gastric Fluid Aspiration in a Rat Model of Lung Transplantation.
Exp Clin Transplant. 17 (1): 84-92. -
Azam, M. et al. (2019) Addition of 2-deoxy-d-ribose to clinically used alginate dressings stimulates angiogenesis and accelerates wound healing in diabetic rats.
J Biomater Appl. 34 (4): 463-75. -
Bhandari, S. et al. (2020) Transcriptome and proteome profiling reveal complementary scavenger and immune features of rat liver sinusoidal endothelial cells and liver macrophages.
BMC Mol Cell Biol. 21 (1): 85. -
Nishida, Y. et al. (2020) Intra-Articular Injection of Stromal Cell-Derived Factor 1α Promotes Meniscal Healing via Macrophage and Mesenchymal Stem Cell Accumulation in a Rat Meniscal Defect Model.
Int J Mol Sci. 21(15):5454. -
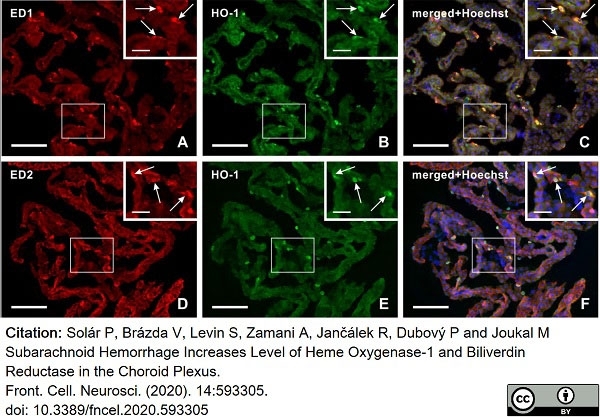
Solár, P. et al. (2020) Subarachnoid Hemorrhage Increases Level of Heme Oxygenase-1 and Biliverdin Reductase in the Choroid Plexus.
Front Cell Neurosci. 14: 593305. -
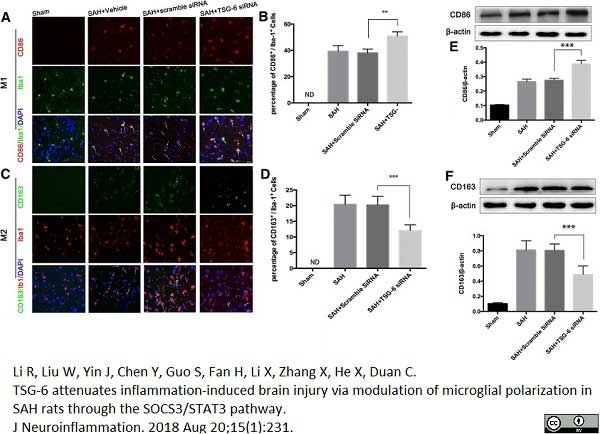
Li, R. et al. (2020) Bone marrow mesenchymal stem cell-derived exosomal microRNA-124-3p attenuates neurological damage in spinal cord ischemia-reperfusion injury by downregulating Ern1 and promoting M2 macrophage polarization.
Arthritis Res Ther. 22 (1): 75. -
Bloomer, S.A. et al. (2020) Aging results in accumulation of M1 and M2 hepatic macrophages and a differential response to gadolinium chloride.
Histochem Cell Biol. 153 (1): 37-48. -
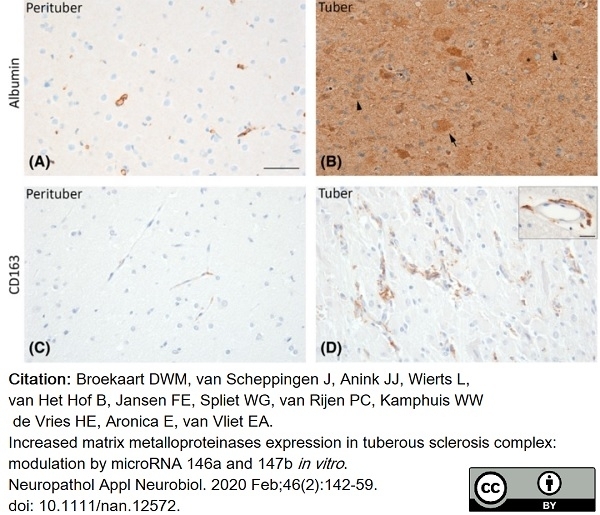
Broekaart, D.W.M. et al. (2020) Increased matrix metalloproteinases expression in tuberous sclerosis complex: modulation by microRNA 146a and 147b in vitro..
Neuropathol Appl Neurobiol. 46 (2): 142-159. -
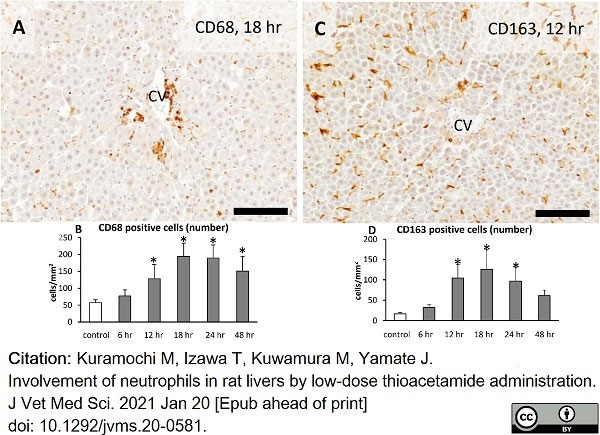
Kuramochi, M. et al. (2021) Involvement of neutrophils in rat livers by low-dose thioacetamide administration.
J Vet Med Sci. 83 (3): 390-396. -
Matsumoto, K. et al. (2021) Role of transient receptor potential vanilloid subtype 2 in lower oesophageal sphincter in rat acid reflux oesophagitis.
J Pharmacol Sci. 146 (3): 125-35. -
Koppe, C. et al. (2021) Local Inflammatory Response after Intramuscularly Implantation of Anti-Adhesive Plasma-Fluorocarbon-Polymer Coated Ti6AI4V Discs in Rats.
Polymers (Basel). 13(16):2684. -
Matsuyama, S. et al. (2021) Properties of macrophages and lymphocytes appearing in rat renal fibrosis followed by repeated injection of cisplatin.
J Vet Med Sci. 83 (9): 1435-42. -
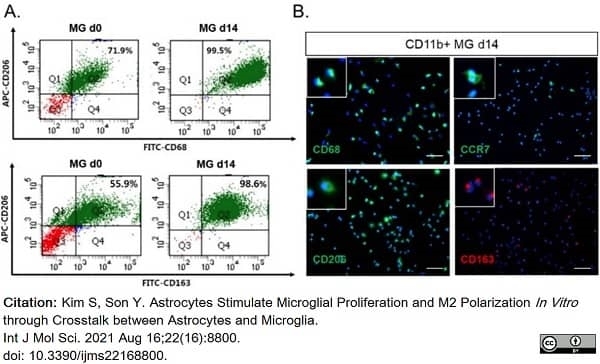
Kim, S. & Son, Y. (2021) Astrocytes Stimulate Microglial Proliferation and M2 Polarization In Vitro. through Crosstalk between Astrocytes and Microglia.
Int J Mol Sci. 22 (16): 8800. -
Zakerkish, F. et al. (2021) Differential effects of the immunosuppressive calcineurin inhibitors cyclosporine-A and tacrolimus on ovulation in a murine model.
Hum Reprod Open. 2021 (2): hoab012. -
Anderson, L.E. et al. (2021) Injection of Micronized Human Amnion/Chorion Membrane Results in Increased Early Supraspinatus Muscle Regeneration in a Chronic Model of Rotator Cuff Tear.
Ann Biomed Eng. 49 (12): 3698-710. -
Hou, Y. et al. (2021) Pseudoginsenoside-F11 promotes functional recovery after transient cerebral ischemia by regulating the microglia/macrophage polarization in rats.
Int Immunopharmacol. 99: 107896. -
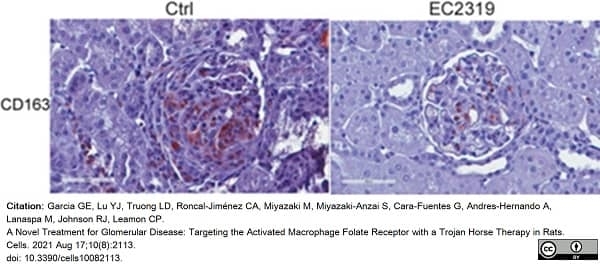
Garcia, G.E. et al. (2021) A Novel Treatment for Glomerular Disease: Targeting the Activated Macrophage Folate Receptor with a Trojan Horse Therapy in Rats.
Cells. 10(8): 2113. -
Pervin, M. et al. (2022) Possible Cytoprotection of Low Dose Lipopolysaccharide in Rat Thioacetamide-Induced Liver Lesions, Focusing on the Analyses of Hepatic Macrophages and Autophagy.
Toxicol Pathol. : 1926233221076758. -
Itoh, M. et al. (2022) Time-series biological responses toward decellularized bovine tendon graft and autograft for 52 consecutive weeks after rat anterior cruciate ligament reconstruction.
Sci Rep. 12 (1): 6751. -
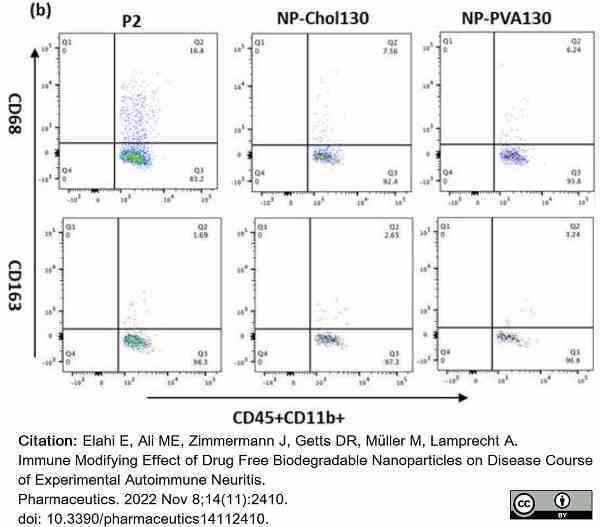
Elahi, E. et al. (2022) Immune Modifying Effect of Drug Free Biodegradable Nanoparticles on Disease Course of Experimental Autoimmune Neuritis.
Pharmaceutics. 14 (11): 2410. -
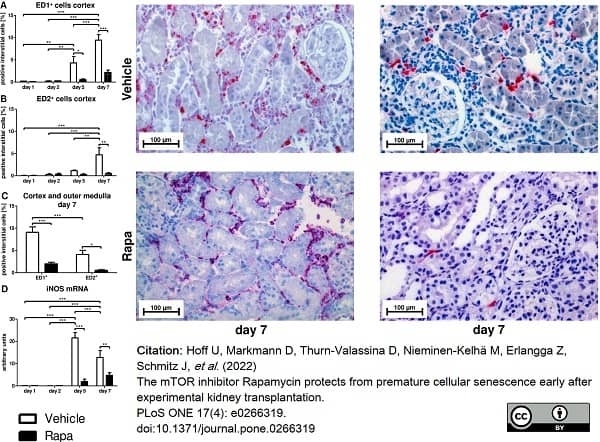
Hoff, U. et al. (2022) The mTOR inhibitor Rapamycin protects from premature cellular senescence early after experimental kidney transplantation.
PLoS One. 17 (4): e0266319. -
Wu, C.C. et al. (2022) Preventive Intrathecal Injection of Bupivacaine Alleviated Microglia Activation and Neuropathic Pain in a Rat Model of Chronic Constriction Injury.
Int J Mol Sci. 23 (13): 7197. -
Köhler, R. et al. (2022) Association of systemic antibody response against polyethylene terephthalate with inflammatory serum cytokine profile following implantation of differently coated vascular prostheses in a rat animal model.
J Biomed Mater Res A. 110 (1): 52-63. -
Takei, R. et al. (2022) Dynamic switch of immunity and antitumor effects of metformin in rat spontaneous esophageal carcinogenesis.
Cancer Immunol Immunother. 71 (4): 777-89. -
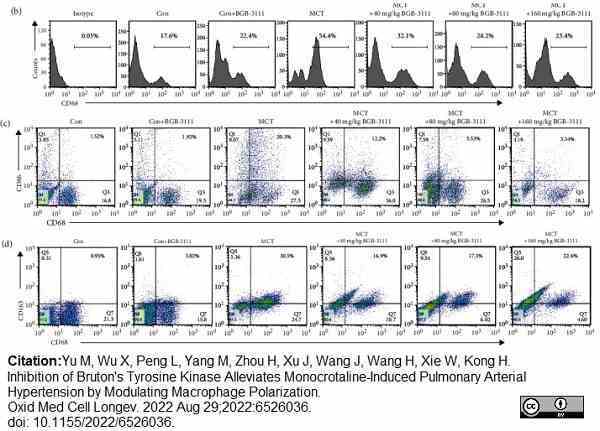
Yu, M. et al. (2022) Inhibition of Bruton's Tyrosine Kinase Alleviates Monocrotaline-Induced Pulmonary Arterial Hypertension by Modulating Macrophage Polarization.
Oxid Med Cell Longev. 2022: 6526036. -
Takami, Y. et al. (2023) The effect of lipopolysaccharide on liver homeostasis and diseases based on the mutual interaction of macrophages, autophagy, and damage-associated molecular patterns in male F344/DuCrlCrlj rats.
Vet Pathol. 60 (4): 461-472. -
Anderson, L.E. et al. (2023) Bone marrow mobilization & local SDF-1α delivery enhances nascent supraspinatus muscle fiber growth.
Tissue Eng Part A. Oct 28 [Epub ahead of print]. -
Xu, Z. et al. (2023) Schwann cells do not promote myogenic differentiation in the EPI loop model.
Tissue Eng Part A. Dec 08 [Epub ahead of print]. -
Bodnar, T.S. et al. (2022) Modulatory role of prenatal alcohol exposure and adolescent stress on the response to arthritis challenge in adult female rats.
EBioMedicine. 77: 103876.
- Synonyms
- ED2
- RRID
- AB_321966
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Rat ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up