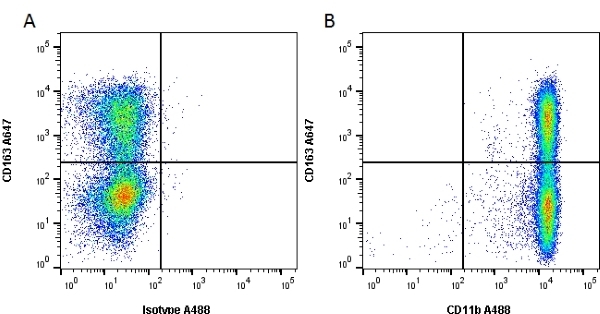
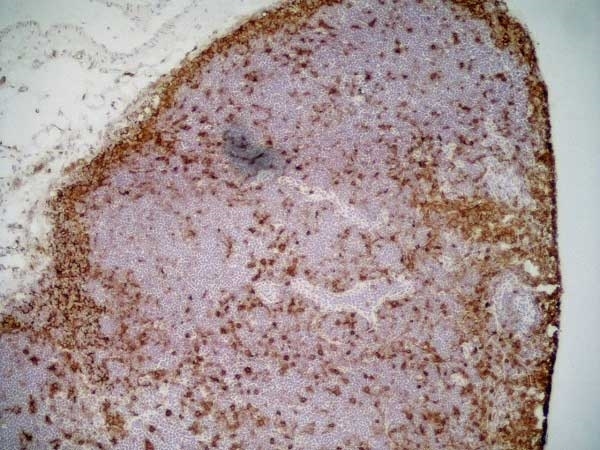
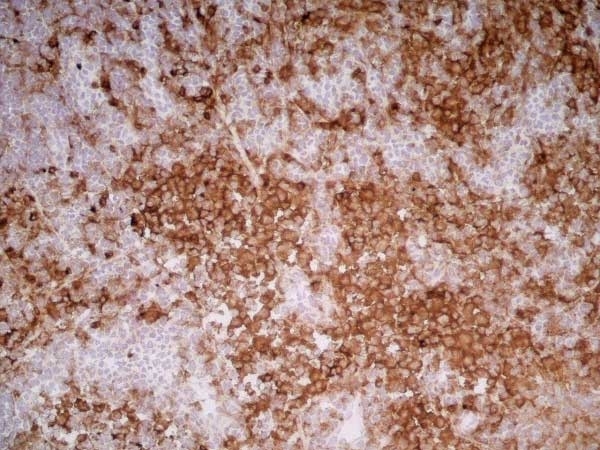
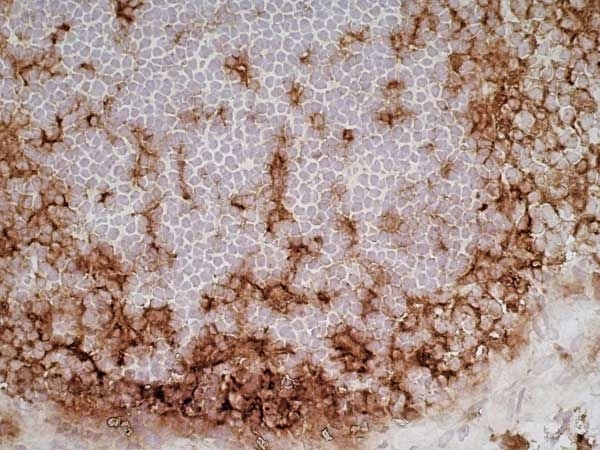
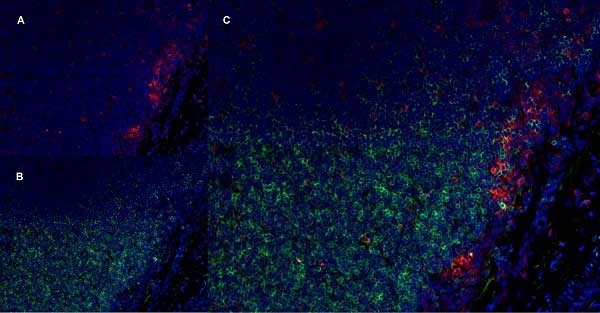
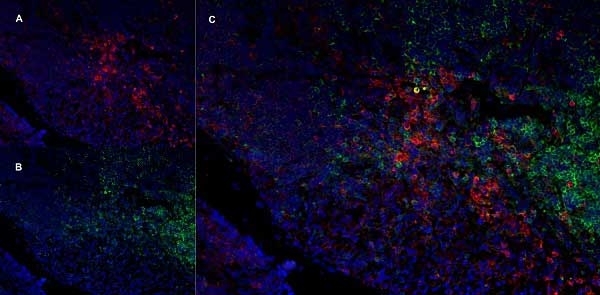
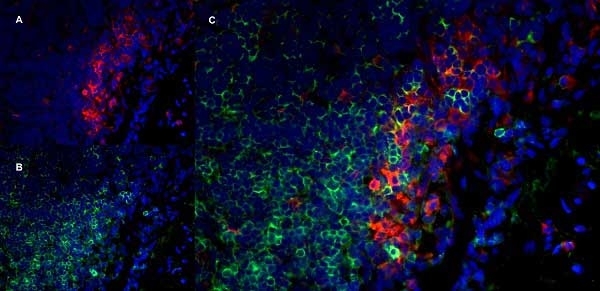
CD11b antibody | OX-42







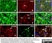

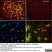



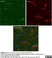
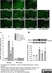
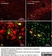
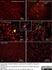
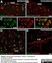
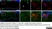
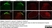


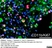

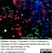

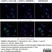













Mouse anti Rat CD11b
- Product Type
- Monoclonal Antibody
- Clone
- OX-42
- Isotype
- IgG2a
- Specificity
- CD11b
| Mouse anti Rat CD11b, clone OX-42 recognizes rat CD11b, also known as integrin alpha-M, the receptor for the iC3b component of complement. CD11b is a 1151 amino acid single pass type 1 transmembrane glycoprotein posessing a single vWFA domain and multiple FG-GAP repeats. CD11b is expressed on most macrophages, including resident and activated peritoneal macrophages and Kupffer cells and around 35% of alveolar macrophages. The antibody also labels dendritic cells, granulocytes and microglia in the brain (Robinson et al.1986). Mouse anti Rat CD11b, clone OX-42 is reported to inhibit complement mediated rosettes (Robinson et al.1986) as well as inhibit myelin binding and uptake (van der Laan et al.1996). |
- Target Species
- Rat
- Product Form
- Purified IgG - liquid
- Preparation
- MCA275GA, MCA275G: Purified IgG prepared by affinity chromatography on Protein A from tissue culture supernatant
- MCA275R: Purified IgG prepared by affinity chromatography on Protein G from tissue culture supernatant
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
0.09% Sodium Azide - Carrier Free
- Yes
- Immunogen
- Resident rat peritoneal macrophages.
- Approx. Protein Concentrations
- IgG concentration 1.0 mg/ml
- Fusion Partners
- Spleen cells from immunized BALB/c mice were fused with cells of the NSO/U mouse myeloma cell line.
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Flow Cytometry | 1/50 | 1/100 | |
| Immunofluorescence | |||
| Immunohistology - Frozen | 1/50 | 1/100 | |
| Immunoprecipitation |
- Flow Cytometry
- Use 10ul of the suggested working dilution to label 106 cells in 100ul.
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Mouse IgG2a Negative Control | MCA1210 | F | 100 Tests |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Mouse IgG2a Negative Control | ||||||
References for CD11b antibody
-
Robinson, A.P. et al. (1986) Macrophage heterogeneity in the rat as delineated by two monoclonal antibodies MRC OX-41 and MRC OX-42, the latter recognizing complement receptor type 3.
Immunology. 57 (2): 239-47. -
Milligan, C.E. et al. (1991) Differential immunochemical markers reveal the normal distribution of brain macrophages and microglia in the developing rat brain.
J Comp Neurol. 314 (1): 125-35. -
Yrjanheikki, J. et al. (1999) A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window.
Proc Natl Acad Sci U S A. 96: 13496-500. -
Draskovic-Pavlovic, B. et al. (1999) Differential effects of anti-rat CD11b monoclonal antibodies on granulocyte adhesiveness.
Immunology. 96: 83-9. -
Kielian, T. and Hickey, W.F. (2000) Proinflammatory cytokine, chemokine, and cellular adhesion molecule expression during the acute phase of experimental brain abscess development.
Am J Pathol. 157: 647-58. -
Choi, S.H. et al. (2003) Thrombin-induced microglial activation produces degeneration of nigral dopaminergic neurons in vivo.
J Neurosci. 23: 5877-86. -
Bruce-Keller, A.J. et al. (2003) Synaptic transport of human immunodeficiency virus-Tat protein causes neurotoxicity and gliosis in rat brain.
J Neurosci. 23: 8417-22. -
Jin, S.X. et al. (2003) p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain.
J Neurosci. 23: 4017-22.
View The Latest Product References
-
Walczak, P. et al. (2004) Do hematopoietic cells exposed to a neurogenic environment mimic properties of endogenous neural precursors?
J Neurosci Res. 76: 244-54. -
Stidworthy, M.F. et al. (2004) Notch1 and Jagged1 are expressed after CNS demyelination, but are not a major rate-determining factor during remyelination.
Brain, 127: 1928-41. -
Foote, A.K. and Blakemore, W.F. (2005) Inflammation stimulates remyelination in areas of chronic demyelination.
Brain. 128: 528-39. -
Jha, P. et al. (2006) The complement system plays a critical role in the development of experimental autoimmune anterior uveitis.
Invest Ophthalmol Vis Sci. 47: 1030-8. -
Clark, A.K. et al (2007) Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain.
Proc Natl Acad Sci U S A. 104: 10655-60. -
Fendrick, S.E. et al. (2007) Formation of multinucleated giant cells and microglial degeneration in rats expressing a mutant Cu/Zn superoxide dismutase gene.
J Neuroinflammation. 4: 9. -
Ji, B. et al. (2008) Imaging of peripheral benzodiazepine receptor expression as biomarkers of detrimental versus beneficial glial responses in mouse models of Alzheimer's and other CNS pathologies.
J Neurosci. 28: 12255-67. -
Jean, Y.H. et al. (2009) Capnellene, a natural marine compound derived from soft coral, attenuates chronic constriction injury-induced neuropathic pain in rats
Br J Pharmacol. 158: 713-25. -
Leonardo, C.C. et al. (2009) Inhibition of gelatinase activity reduces neural injury in an ex vivo model of hypoxia-ischemia.
Neuroscience. 160: 755-66. -
Jin, Y. et al. (2009) Mast cells are early responders after hypoxia-ischemia in immature rat brain.
Stroke. 40: 3107-12. -
Baca Jones, C.C. (2009) Rat cytomegalovirus infection depletes MHC II in bone marrow derived dendritic cells.
Virology. 388: 78-90. -
Schlichter, L.C. et al. (2010) The Ca2+ activated SK3 channel is expressed in microglia in the rat striatum and contributes to microglia-mediated neurotoxicity in vitro.
J Neuroinflammation. 7: 4. -
Pickard, M.R. and Chari, D.M. (2010) Robust uptake of magnetic nanoparticles (MNPs) by central nervous system (CNS) microglia: Implications for particle uptake in mixed neural cell populations.
Int J Mol Sci. 11: 967-81. -
Medders, K.E. et al. (2010) Activation of p38 MAPK is required in monocytic and neuronal cells for HIV glycoprotein 120-induced neurotoxicity.
J Immunol. 185: 4883-95. -
Li, K. et al. (2010) Systemic minocycline differentially influences changes in spinal microglial markers following formalin-induced nociception.
J Neuroimmunol. 221: 25-31. -
Su, D. et al. (2010) Lidocaine attenuates proinflammatory cytokine production induced by extracellular adenosine triphosphate in cultured rat microglia.
Anesth Analg. 111: 768-74. -
Jokic, N. et al. (2010) The human G93A-SOD1 mutation in a pre-symptomatic rat model of amyotrophic lateral sclerosis increases the vulnerability to a mild spinal cord compression.
BMC Genomics. 11: 633. -
Zhou, D. et al. (2010) Involvement of spinal microglial P2X7 receptor in generation of tolerance to morphine analgesia in rats.
J Neurosci. 30: 8042-7. -
Smith, J.S. et al. (2010) Role of Early Surgical Decompression of the Intradural Space After Cervical Spinal Cord Injury in an Animal Model
J Bone Joint Surg Am. 92: 1206-14. -
Jiang, Y. et al. (2010) Effects of brain-derived neurotrophic factor on local inflammation in experimental stroke of rat.
Mediators Inflamm. 2010: 372423. -
Graber, D.J. et al. (2010) Progressive changes in microglia and macrophages in spinal cord and peripheral nerve in the transgenic rat model of amyotrophic lateral sclerosis.
J Neuroinflammation. 7: 8. -
Jeong, H.K. et al. (2010) Inflammatory responses are not sufficient to cause delayed neuronal death in ATP-induced acute brain injury.
PLoS One. 5: e13756. -
Wu, J. et al. (2010) Interaction of NG2(+) glial progenitors and microglia/macrophages from the injured spinal cord.
Glia. 58: 410-22. -
Guo, W. et al. (2010) Long lasting pain hypersensitivity following ligation of the tendon of the masseter muscle in rats: a model of myogenic orofacial pain.
Mol Pain. 6: 40. -
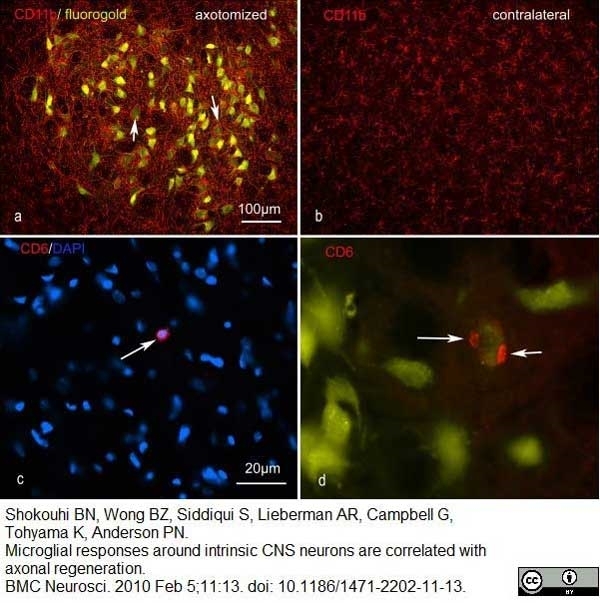
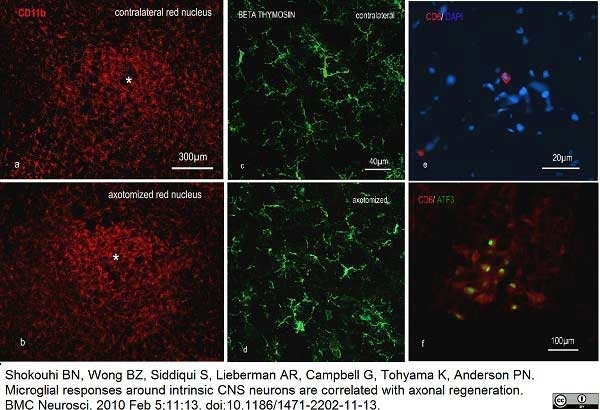
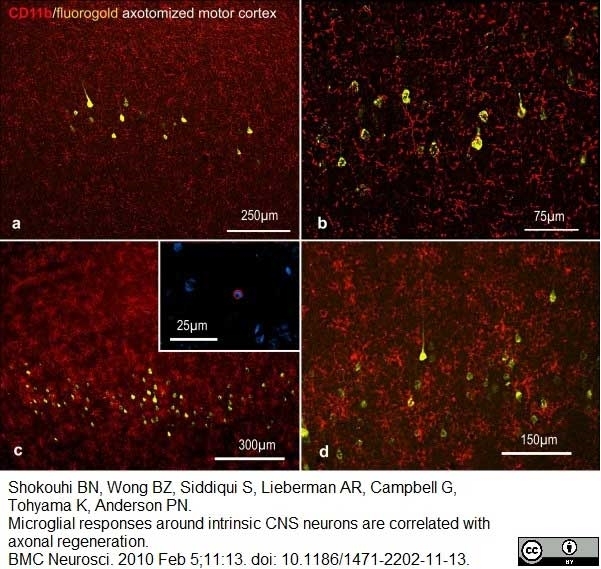
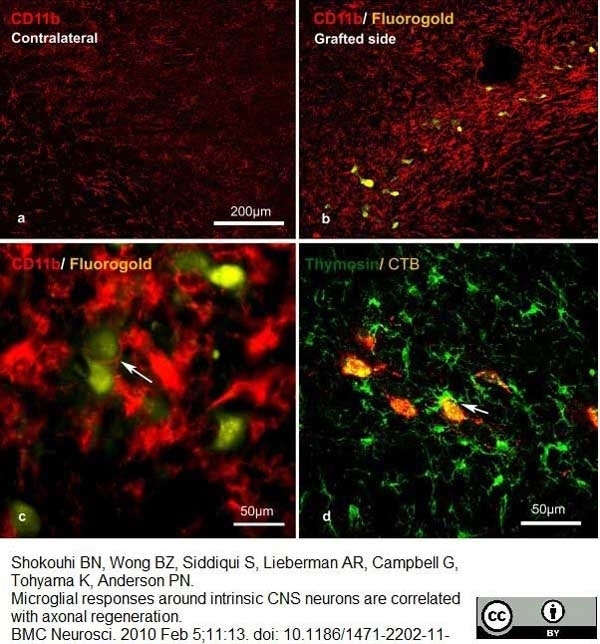
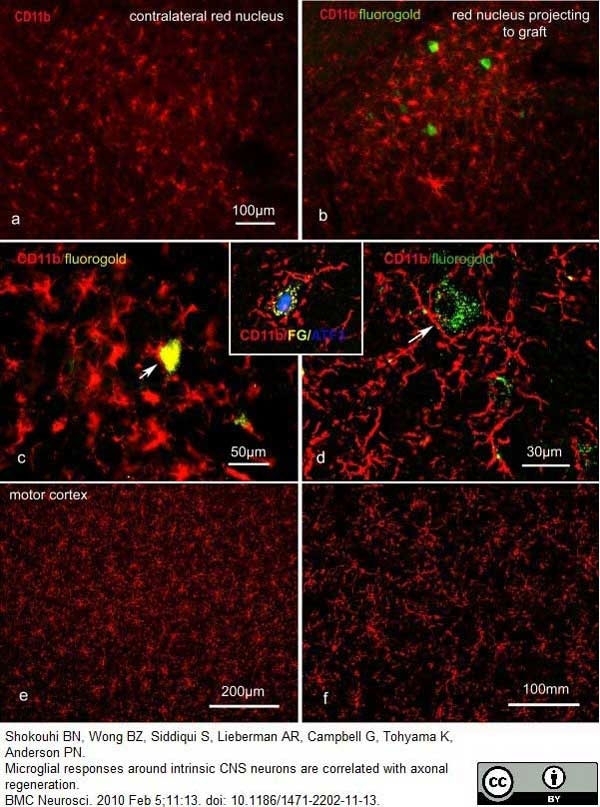
Shokouhi, B.N. et al (2010) Microglial responses around intrinsic CNS neurons are correlated with axonal regeneration.
BMC Neurosci. 11: 13. -
Calvo, M. et al. (2010) Neuregulin-ErbB signaling promotes microglial proliferation and chemotaxis contributing to microgliosis and pain after peripheral nerve injury.
J Neurosci. 30 (15): 5437-50. -
Chew, S.S. et al. (2011) Response of retinal Connexin43 to optic nerve injury.
Invest Ophthalmol Vis Sci. 52: 3620-9. -
Spencer-Segal, J.L. et al. (2011) Distribution of Phosphorylated TrkB Receptor in the Mouse Hippocampal Formation Depends on Sex and Estrous Cycle Stage.
J Neurosci. 31: 6780-90. -
Converse, A.K. et al. (2011) 11C-(R)-PK11195 PET imaging of microglial activation and response to minocycline in zymosan-treated rats.
J Nucl Med. 52: 257-62. -
Feng, Y. et al (2011) Gene expression profiling of vasoregression in the retina--involvement of microglial cells.
PLoS One. 6(2): e16865. -
Szmydynger-Chodobska, J. et al. (2011) Multiple sites of vasopressin synthesis in the injured brain.
J Cereb Blood Flow Metab. 31: 47-51. -
Toda, S. et al. (2011) A local anesthetic, ropivacaine, suppresses activated microglia via a nerve growth factor-dependent mechanism and astrocytes via a nerve growth factor-independent mechanism in neuropathic pain.
Mol Pain. 7: 2. -
Xu, Q. et al. (2011) Spinal phosphinositide 3-kinase-Akt-mammalian target of rapamycin signaling cascades in inflammation-induced hyperalgesia.
J Neurosci. 31: 2113-24. -
Morales-Garcia, J.A. et al. (2011) Phosphodiesterase 7 inhibition preserves dopaminergic neurons in cellular and rodent models of Parkinson disease.
PLoS One. 6(2):e17240. -
Signarovitz, A.L. et al. (2012) Mucosal immunization with live attenuated Francisella novicida U112ΔiglB protects against pulmonary F. tularensis SCHU S4 in the Fischer 344 rat model.
PLoS One. 7: e47639. -
Ortega, F.J. et al. (2012) Glibenclamide enhances neurogenesis and improves long-term functional recovery after transient focal cerebral ischemia.
J Cereb Blood Flow Metab. 33: 356-64. -
Lavisse, S. et al. (2012) Reactive astrocytes overexpress TSPO and are detected by TSPO positron emission tomography imaging.
J Neurosci. 32: 10809-18. -
Schonberg, D.L. et al. (2012) Ferritin stimulates oligodendrocyte genesis in the adult spinal cord and can be transferred from macrophages to NG2 cells in vivo.
J Neurosci. 32: 5374-84. -
d'Avila, J.C. et al. (2012) Microglial activation induced by brain trauma is suppressed by post-injury treatment with a PARP inhibitor.
J Neuroinflammation. 9: 31. -
Lovett-Barr, M.R. et al. (2012) Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury.
J Neurosci. 32 (11): 3591-600. -
Tchoukalova, Y.D. et al. (2012) In vivo adipogenesis in rats measured by cell kinetics in adipocytes and plastic-adherent stroma-vascular cells in response to high-fat diet and thiazolidinedione.
Diabetes. 61: 137-44. -
Zhang, Z.J. et al. (2012) Chemokine CCL2 and its receptor CCR2 in the medullary dorsal horn are involved in trigeminal neuropathic pain.
J Neuroinflammation. 9: 136. -
Liew, H.K. et al. (2012) Systemic administration of urocortin after intracerebral hemorrhage reduces neurological deficits and neuroinflammation in rats.
J Neuroinflammation. 9: 13. -
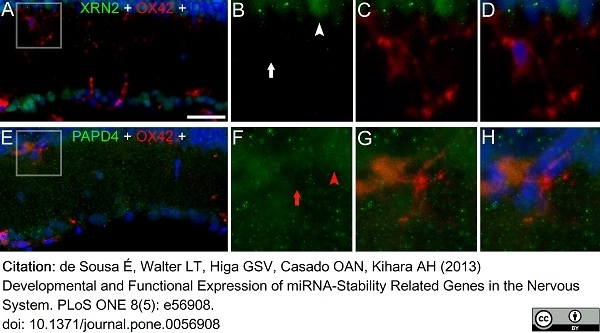
de Sousa, É et al. (2013) Developmental and functional expression of miRNA-stability related genes in the nervous system.
PLoS One. 8 (5): e56908. -
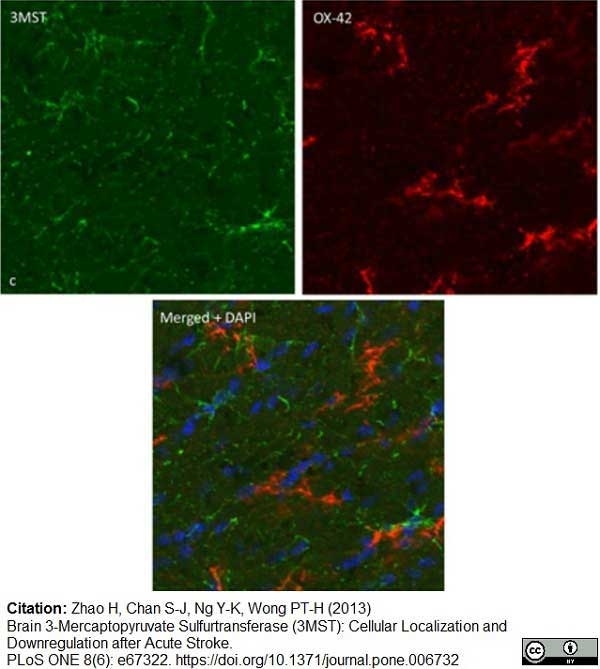
Zhao, H. et al. (2013) Brain 3-Mercaptopyruvate Sulfurtransferase (3MST): Cellular Localization and Downregulation after Acute Stroke.
PLoS One. 8(6):e67322. -
Huang, S. et al. (2015) Expression of Peroxiredoxin 1 After Traumatic Spinal Cord Injury in Rats.
Cell Mol Neurobiol. 35 (8): 1217-26. -
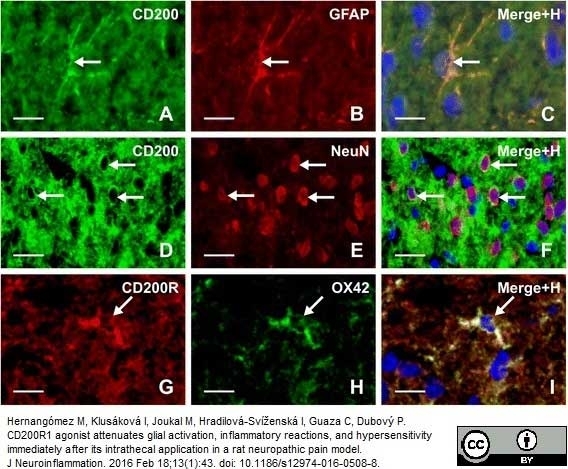
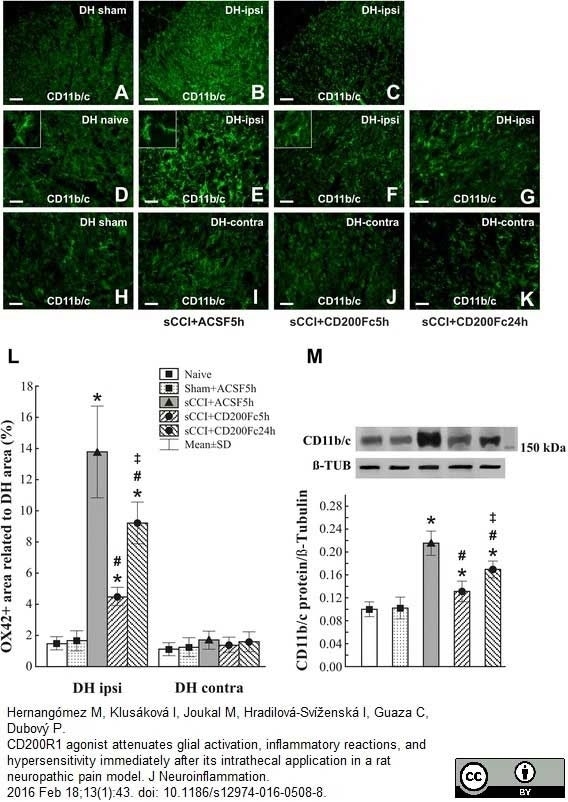
Hernangómez M et al. (2016) CD200R1 agonist attenuates glial activation, inflammatory reactions, and hypersensitivity immediately after its intrathecal application in a rat neuropathic pain model.
J Neuroinflammation. 13 (1): 43. -
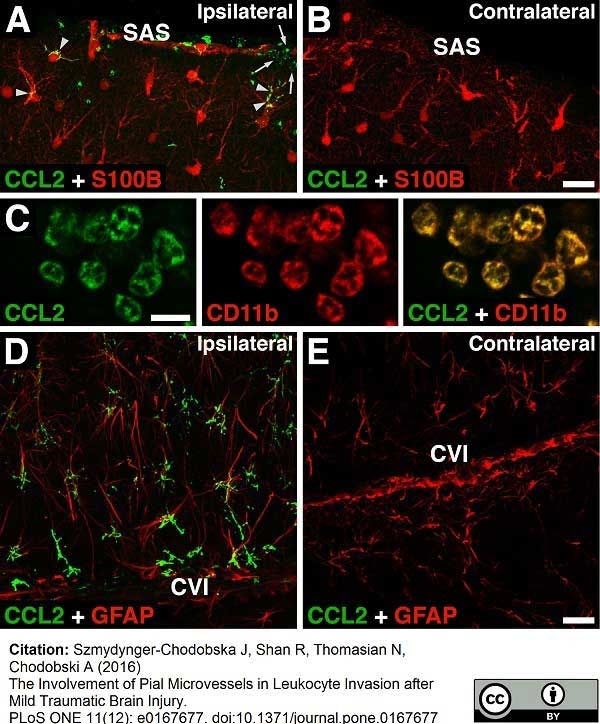
Szmydynger-Chodobska, J. et al. (2016) The Involvement of Pial Microvessels in Leukocyte Invasion after Mild Traumatic Brain Injury.
PLoS One. 11 (12): e0167677. -
Liu, Z. et al. (2016) Leukocyte Infiltration Triggers Seizure Recurrence in a Rat Model of Temporal Lobe Epilepsy.
Inflammation. 39 (3): 1090-8. -
Chen, X. et al. (2017) TGF-β1 Neuroprotection via Inhibition of Microglial Activation in a Rat Model of Parkinson's Disease.
J Neuroimmune Pharmacol. 12 (3): 433-46. -
Popiolek-Barczyk, K. et al. (2017) Biphalin, a Dimeric Enkephalin, Alleviates LPS-Induced Activation in Rat Primary Microglial Cultures in Opioid Receptor-Dependent and Receptor-Independent Manners.
Neural Plast. 2017: 3829472. -
Huang RY et al. (2017) Rapid and Delayed Effects of Pulsed Radiofrequency on Neuropathic Pain: Electrophysiological, Molecular, and Behavioral Evidence Supporting Long-Term Depression.
Pain Physician. 20 (2): E269-E283. -
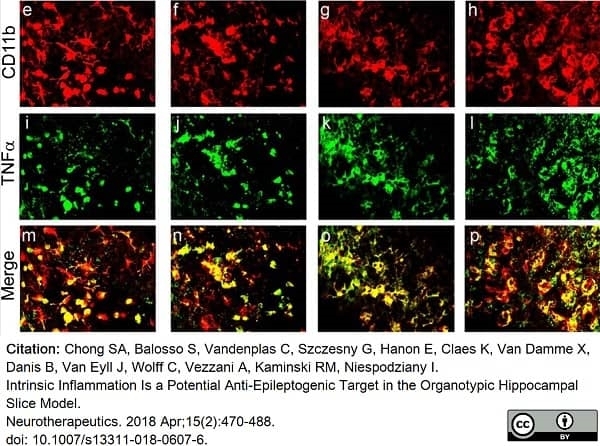
Chong, S.A. et al. (2018) Intrinsic Inflammation Is a Potential Anti-Epileptogenic Target in the Organotypic Hippocampal Slice Model.
Neurotherapeutics. 15 (2): 470-88. -
Terayama, R. et al. (2018) A3 adenosine receptor agonist attenuates neuropathic pain by suppressing activation of microglia and convergence of nociceptive inputs in the spinal dorsal horn.
Exp Brain Res. 236 (12): 3203-13. -
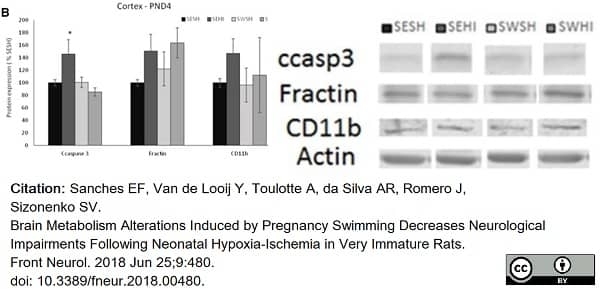
Sanches, E.F. et al. (2018) Brain Metabolism Alterations Induced by Pregnancy Swimming Decreases Neurological Impairments Following Neonatal Hypoxia-Ischemia in Very Immature Rats.
Front Neurol. 9: 480. -
Collins, J.J.P. et al. (2018) Impaired Angiogenic Supportive Capacity and Altered Gene Expression Profile of Resident CD146+ Mesenchymal Stromal Cells Isolated from Hyperoxia-Injured Neonatal Rat Lungs.
Stem Cells Dev. 27 (16): 1109-24. -
Wang, Z.C. et al. (2018) Involvement of NF-κB and the CX3CR1 Signaling Network in Mechanical Allodynia Induced by Tetanic Sciatic Stimulation.
Neurosci Bull. 34 (1): 64-73. -
Bourke, G. et al. (2018) Effects of early nerve repair on experimental brachial plexus injury in neonatal rats.
J Hand Surg Eur Vol. 43 (3): 275-81. -
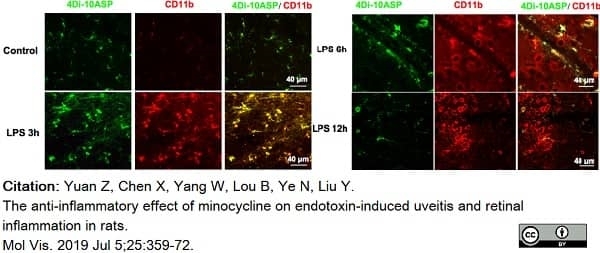
Yuan, Z. et al. (2019) The anti-inflammatory effect of minocycline on endotoxin-induced uveitis and retinal inflammation in rats.
Mol Vis. 25: 359-72. -
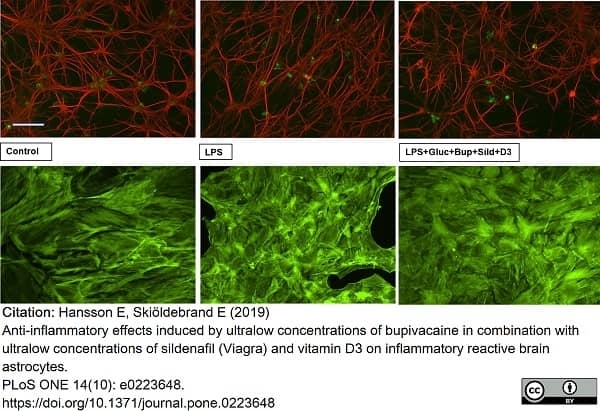
Hansson, E. & Skiöldebrand, E. (2019) Anti-inflammatory effects induced by ultralow concentrations of bupivacaine in combination with ultralow concentrations of sildenafil (Viagra) and vitamin D3 on inflammatory reactive brain astrocytes.
PLoS One. 14 (10): e0223648. -
Muratori, L. et al. (2019) New basic insights on the potential of a chitosan-based medical device for improving functional recovery after radical prostatectomy.
BJU Int. 124 (6): 1063-76. -
Hahm, S.C. et al. (2019) Transcutaneous Electrical Nerve Stimulation Reduces Knee Osteoarthritic Pain by Inhibiting Spinal Glial Cells in Rats.
Phys Ther. 99 (9): 1211-23. -
Hellenbrand, D.J. et al. (2019) Sustained interleukin-10 delivery reduces inflammation and improves motor function after spinal cord injury.
J Neuroinflammation. 16 (1): 93. -
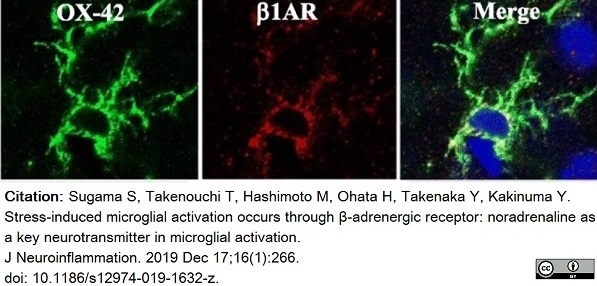
Sugama, S. et al. (2019) Stress-induced microglial activation occurs through β-adrenergic receptor: noradrenaline as a key neurotransmitter in microglial activation.
J Neuroinflammation. 16 (1): 266. -
Anqi, X. et al. (2019) Neuroprotective potential of GDF11 in experimental intracerebral hemorrhage in elderly rats.
J Clin Neurosci. 63: 182-8. -
Klemm, P. et al. (2019) Hypothermia protects retinal ganglion cells against hypoxia-induced cell death in a retina organ culture model.
Clin Exp Ophthalmol. 47 (8): 1043-54. -
Allendorf, D.H. et al. (2020) Activated microglia desialylate their surface, stimulating complement receptor 3-mediated phagocytosis of neurons.
Glia. 68 (5): 989-998. -
Cohrs, G. et al. (2020) Expression Patterns of Hypoxia-Inducible Factors, Proinflammatory, and Neuroprotective Cytokines in Neuroepithelial Tissues of Lumbar Spinal Lipomas-A Pilot Study.
World Neurosurg. 141: e633-e644. -
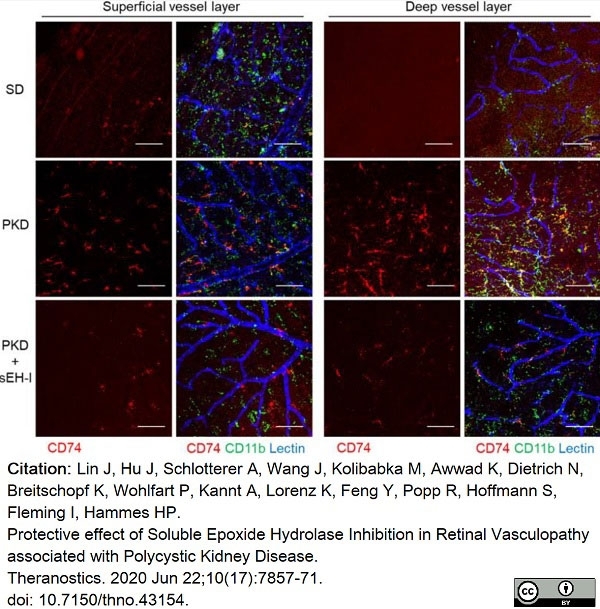
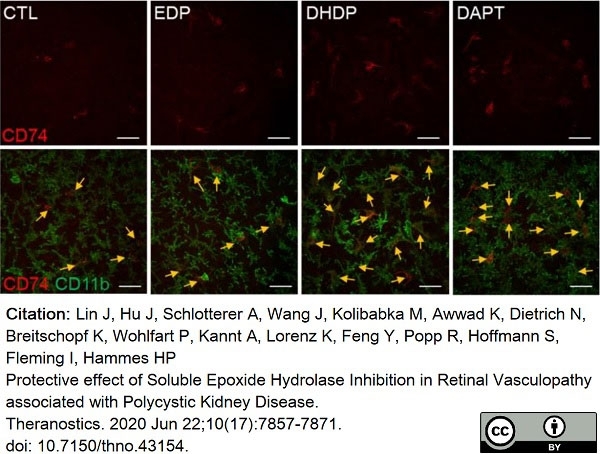
Lin, J. et al. (2020) Protective effect of Soluble Epoxide Hydrolase Inhibition in Retinal Vasculopathy associated with Polycystic Kidney Disease.
Theranostics. 10 (17): 7857-71. -
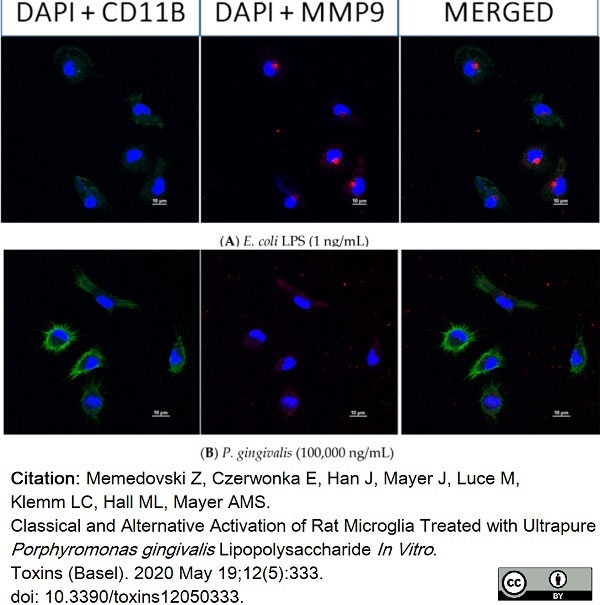
Memedovski, Z. et al. (2020) Classical and Alternative Activation of Rat Microglia Treated with Ultrapure Porphyromonas gingivalis Lipopolysaccharide In Vitro..
Toxins (Basel). 12(5): 333. -
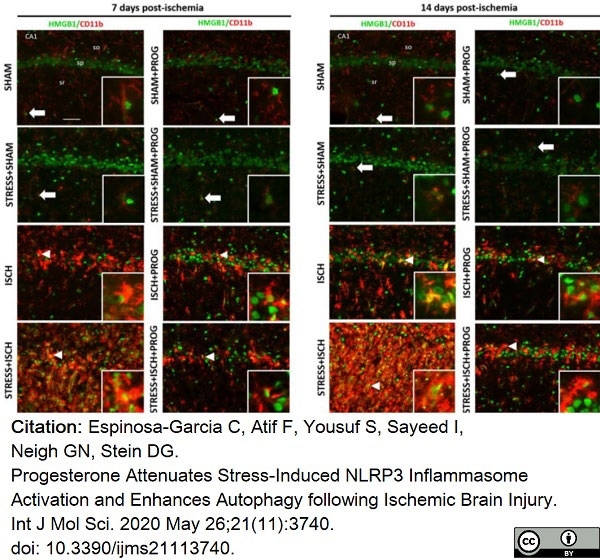
Espinosa-Garcia, C. et al. (2020) Progesterone Attenuates Stress-Induced NLRP3 Inflammasome Activation and Enhances Autophagy following Ischemic Brain Injury.
Int J Mol Sci. 21 (11): 3740. -
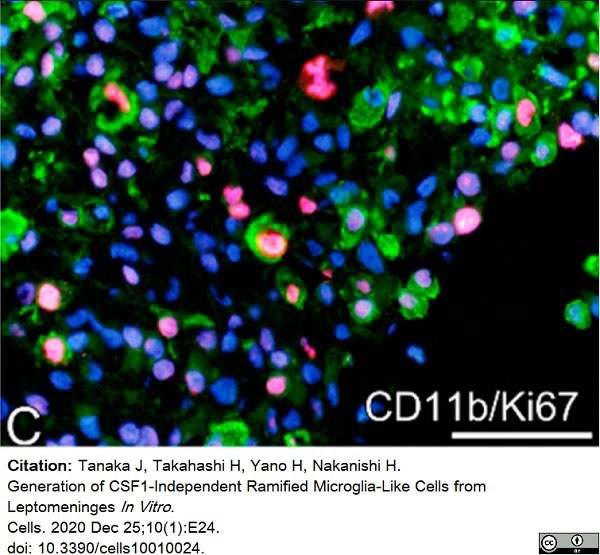
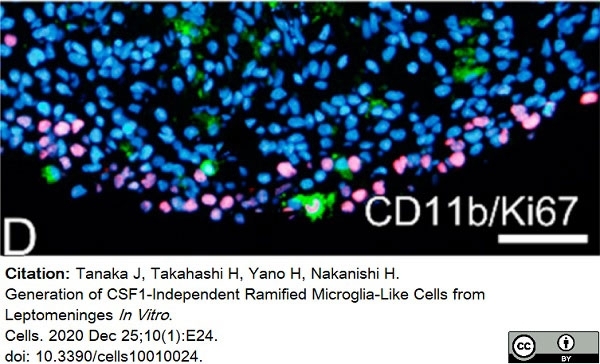
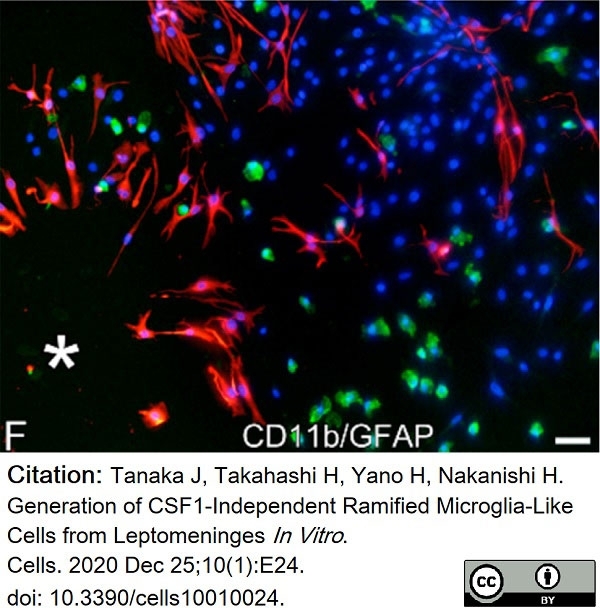
Tanaka, J. et al. (2020) Generation of CSF1-Independent Ramified Microglia-Like Cells from Leptomeninges In Vitro.
Cells. 10 (1): 24. -
van Vliet, E.A. (2020) Long-lasting blood-brain barrier dysfunction and neuroinflammation after traumatic brain injury
Neurobiol Dis 11:13. -
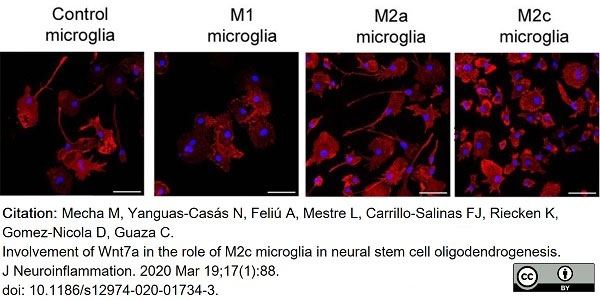
Mecha, M. et al. (2020) Involvement of Wnt7a in the role of M2c microglia in neural stem cell oligodendrogenesis.
J Neuroinflammation. 17 (1): 88. -
Chun, S. et al. (2021) The Peripheral Role of CCL2 in the Anti-Nociceptive Effect of Sigma-1 Receptor Antagonist BD1047 on Inflammatory Hyperalgesia in Rats.
Int J Mol Sci. 22(21):11730. -
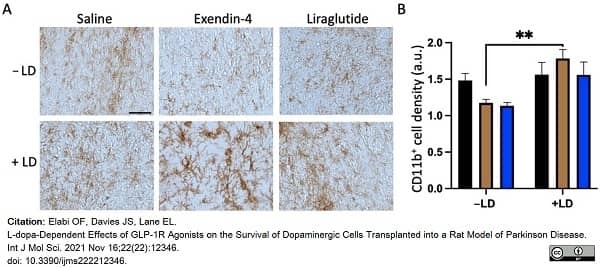
Elabi, O.F. et al. (2021) L-dopa-Dependent Effects of GLP-1R Agonists on the Survival of Dopaminergic Cells Transplanted into a Rat Model of Parkinson Disease.
Int J Mol Sci. 22(22):12346. -
Zhang, J. et al. (2021) Significant higher-level C-C motif chemokine ligand 2/3 and chemotactic power in cerebral white matter than grey matter in rat and human.
Eur J Neurosci. 54 (1): 4088-100. -
Sato, T. et al. (2021) Distribution of alpha-synuclein in the rat cranial sensory ganglia, and oro-cervical regions.
Ann Anat. 238: 151776. -
Winkler, A. et al. (2021) Blood-brain barrier resealing in neuromyelitis optica occurs independently of astrocyte regeneration.
J Clin Invest. 131(5):e141694. -
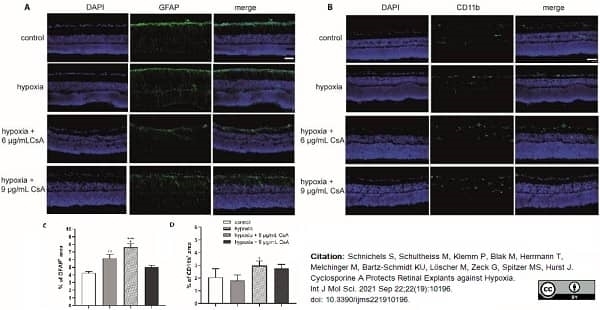
Schnichels, S. et al. (2021) Cyclosporine A Protects Retinal Explants against Hypoxia.
Int J Mol Sci. 22 (19):10196. -
Valenzuela, R. et al. (2021) An ACE2/Mas-related receptor MrgE axis in dopaminergic neuron mitochondria.
Redox Biol. 46: 102078. -
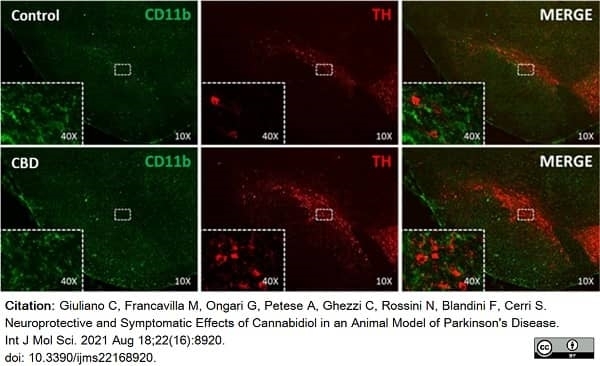
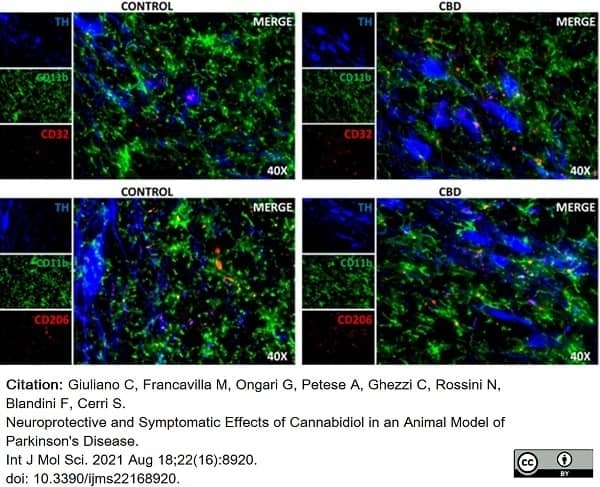
Giuliano, C. et al. (2021) Neuroprotective and Symptomatic Effects of Cannabidiol in an Animal Model of Parkinson's Disease.
Int J Mol Sci. 22 (16): 8920. -
Hosoi, R. et al. (2021) Evaluation of intracellular processes in quinolinic acid-induced brain damage by imaging reactive oxygen species generation and mitochondrial complex I activity.
EJNMMI Res. 11 (1): 99. -
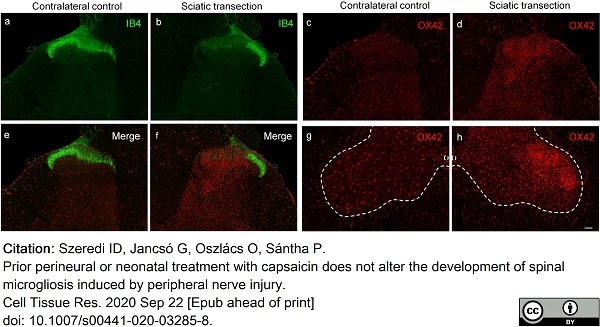
Szeredi, I.D. et al. (2021) Prior perineural or neonatal treatment with capsaicin does not alter the development of spinal microgliosis induced by peripheral nerve injury.
Cell Tissue Res. 383 (2): 677-92. -
Kuo, T.T. et al. (2021) Post-stroke Delivery of Valproic Acid Promotes Functional Recovery and Differentially Modifies Responses of Peri-Infarct Microglia.
Front Mol Neurosci. 14: 639145. -
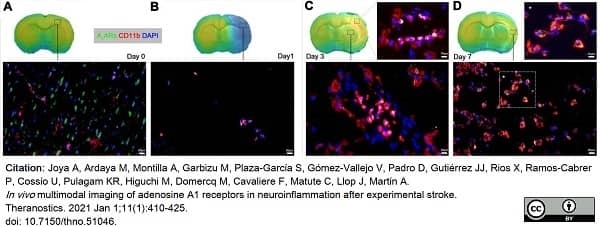
Joya, A. et al. (2021) In vivo multimodal imaging of adenosine A1 receptors in neuroinflammation after experimental stroke.
Theranostics. 11 (1): 410-25. -
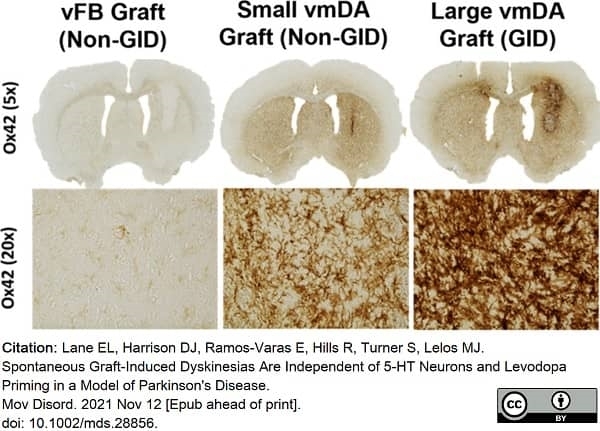
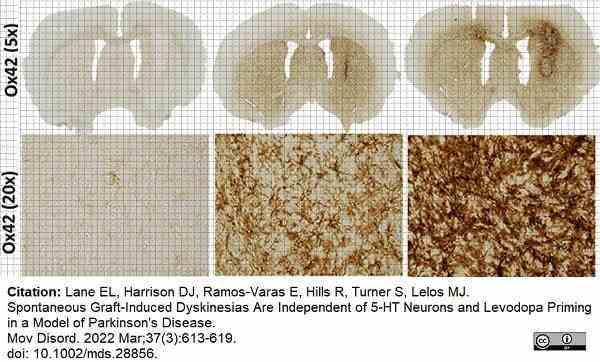
Lane, E.L. et al. (2022) Spontaneous Graft-Induced Dyskinesias Are Independent of 5-HT Neurons and Levodopa Priming in a Model of Parkinson's Disease.
Mov Disord. 37 (3): 613-9. -
Kuter, K.Z. et al. (2022) The influence of preconditioning with low dose of LPS on paraquat-induced neurotoxicity, microglia activation and expression of α-synuclein and synphilin-1 in the dopaminergic system.
Pharmacol Rep. 74 (1): 67-83. -
Huang, S. et al. (2022) Hydrogen sulfide supplement preserves mitochondrial function of retinal ganglion cell in a rat glaucoma model.
Cell Tissue Res. 389 (2): 171-85. -
Dias, L. et al. (2022) Aβ1-42 peptides blunt the adenosine A2A receptor-mediated control of the interplay between P2X7 and P2Y1 receptors mediated calcium responses in astrocytes.
Cell Mol Life Sci. 79 (8): 457. -
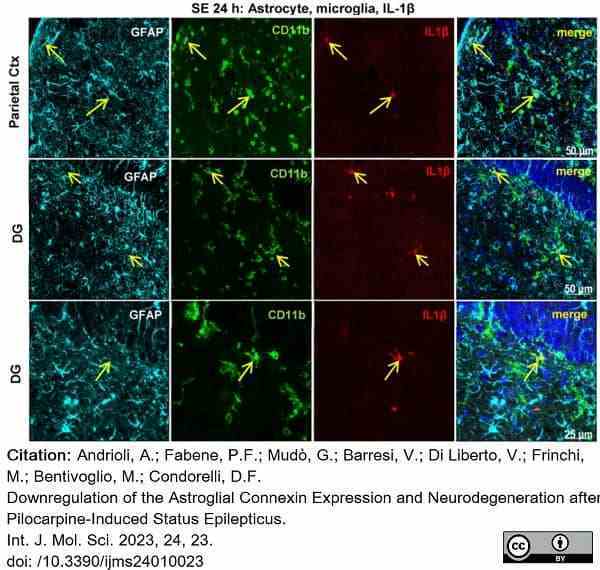
Andrioli, A. et al. (2022) Downregulation of the Astroglial Connexin Expression and Neurodegeneration after Pilocarpine-Induced Status Epilepticus.
Int J Mol Sci. 24 (1): 23. -
Li, H. et al. (2023) Cellular Localization and Distribution of TGF-β1, GDNF and PDGF-BB in the Adult Primate Central Nervous System.
Neurochem Res. 48 (8): 2406-23. -
Li, Li-H. et al. (2023) Ovariectomy induces hyperalgesia accompanied by upregulated estrogen receptor α and protein kinase B in the rat spinal cord
Physiol Behav. 271:114342. -
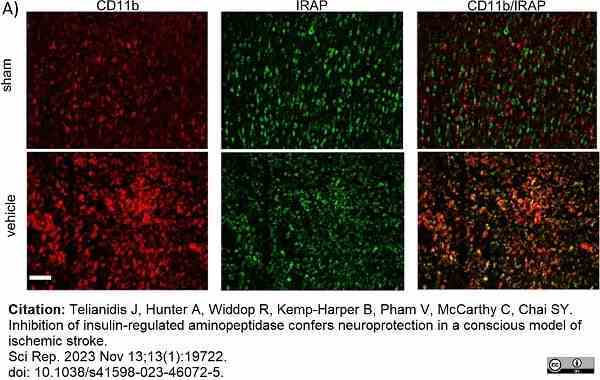
Telianidis, J. et al. (2023) Inhibition of insulin-regulated aminopeptidase confers neuroprotection in a conscious model of ischemic stroke.
Sci Rep. 13 (1): 19722. -
Khayrullina, G. et al. (2023) Differential effects of NOX2 and NOX4 inhibition after rodent spinal cord injury.
PLoS One. 18 (3): e0281045. -
Moretti, M. et al. (2023) "Combo" Multi-Target Pharmacological Therapy and New Formulations to Reduce Inflammation and Improve Endogenous Remyelination in Traumatic Spinal Cord Injury.
Cells. 12 (9): 1331. -
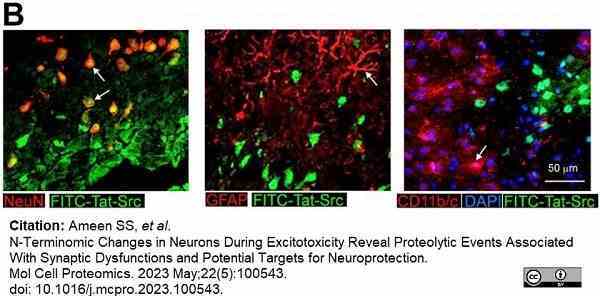
Ameen, S.S. et al. (2023) N-Terminomic Changes in Neurons During Excitotoxicity Reveal Proteolytic Events Associated With Synaptic Dysfunctions and Potential Targets for Neuroprotection.
Mol Cell Proteomics. 22 (5): 100543. -
Sinha, S. et al. (2020) Maternal Spirulina supplementation during pregnancy and lactation partially prevents oxidative stress, glial activation and neuronal damage in protein malnourished F1 progeny.
Neurochem Int. 141: 104877. -
Huang, C.T. et al. (2018) Erythropoietin reduces nerve demyelination, neuropathic pain behavior and microglial MAPKs activation through erythropoietin receptors on Schwann cells in a rat model of peripheral neuropathy.
Glia. 66 (11): 2299-315. -
Routhe, L.J. et al. (2020) Astrocytic expression of ZIP14 (SLC39A14) is part of the inflammatory reaction in chronic neurodegeneration with iron overload.
Glia. 68 (9): 1810-23. -
Moriyama, M. et al. (2018) S-Equol, a Major Isoflavone from Soybean, Inhibits Nitric Oxide Production in Lipopolysaccharide-Stimulated Rat Astrocytes Partially via the GPR30-Mediated Pathway.
Int J Inflam. 2018: 8496973. -
Jeon, M.T. et al. (2020) Neurotrophic interactions between neurons and astrocytes following AAV1-Rheb(S16H) transduction in the hippocampus in vivo.
Br J Pharmacol. 177 (3): 668-686. -
Li, X. et al. (2019) Magnesium sulfate attenuates brain edema by lowering AQP4 expression and inhibits glia-mediated neuroinflammation in a rodent model of eclampsia.
Behav Brain Res. 364: 403-12. -
Li, Q. et al. (2019) Spinal IL-36γ/IL-36R participates in the maintenance of chronic inflammatory pain through astroglial JNK pathway.
Glia. 67 (3): 438-451. -
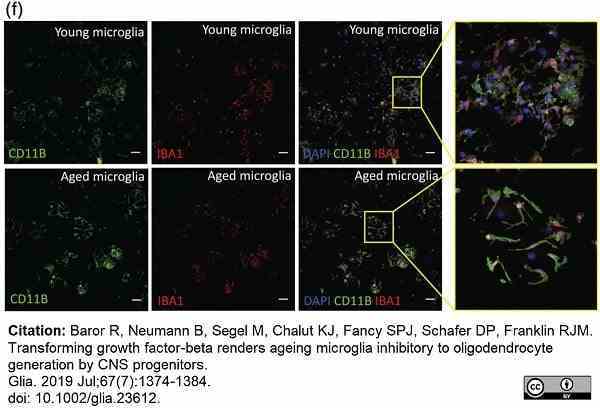
Baror, R. et al. (2019) Transforming growth factor-beta renders ageing microglia inhibitory to oligodendrocyte generation by CNS progenitors.
Glia. 67 (7): 1374-84. -
Lane, E.L. et al. (2022) Spontaneous Graft-Induced Dyskinesias Are Independent of 5-HT Neurons and Levodopa Priming in a Model of Parkinson's Disease.
Mov Disord. 37 (3): 613-9. -
Ekong, M.B. et al. (2024) EVALUATION OF PRENATAL CALABASH CHALK GEOPHAGY ON THE DEVELOPING BRAIN OF WISTAR RATS
IBRO Neuroscience Reports. 14 Mar [Epub ahead of print]. -
Demartini, C. et al. (2022) Modulation of Glia Activation by TRPA1 Antagonism in Preclinical Models of Migraine.
Int J Mol Sci. 23 (22):14085
- Synonyms
- Integrin Alpha M Chain
- MAC-1
- RRID
- AB_321301
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Rat ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up