WC1 antibody | CC15




Mouse anti Bovine WC1
- Product Type
- Monoclonal Antibody
- Clone
- CC15
- Isotype
- IgG2a
- Specificity
- WC1
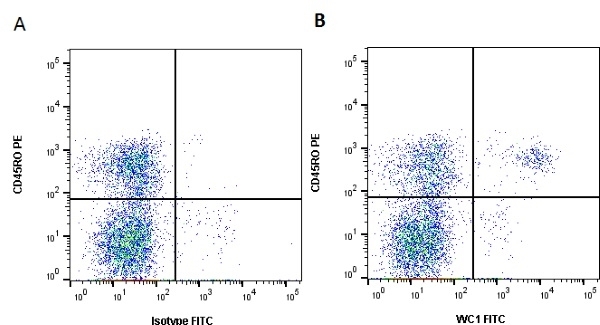
| Mouse anti Bovine WC1 antibody, clone CC15 recognizes bovine WC1. WC1 is a ~215/300 kDa antigen expressed on the majority of gamma/delta T lymphocytes. These cells also express low levels of CD5, but are negative for other B and T cell markers. The WC1 antigen is reported to be involved in a number of processes, including activation of gamma/delta T cells and the development of a Th1-biased acquired immune response (Rogers et al. 2005). Mouse anti Bovine WC1 antibody, clone CC15 is routinely tested in flow cytometry on bovine peripheral blood lymphocytes. |
- Target Species
- Bovine
- Species Cross-Reactivity
-
Target Species Cross Reactivity Sheep Goat - N.B. Antibody reactivity and working conditions may vary between species.
- Product Form
- Purified IgG - liquid
- Preparation
- Purified IgG prepared by affinity chromatography on Protein G from tissue culture supernatant
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
0.09% Sodium Azide - Carrier Free
- Yes
- Immunogen
- Bovine lymphocytes
- Approx. Protein Concentrations
- IgG concentration 1.0 mg/ml
- Fusion Partners
- Spleen cells from immunised BALB/c mice were fused with cells of the mouse NS1 myeloma cell line
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Flow Cytometry | 1/25 | 1/200 | |
| Immunohistology - Frozen | |||
| Immunohistology - Paraffin 1 | |||
| Immunoprecipitation |
- 1This product requires protein digestion pre-treatment of paraffin sections e.g. trypsin or pronase. Overnight incubation with clone CC15 is recommended.
- Flow Cytometry
- Use 10ul of the suggested working dilution to label 106 cells in 100ul
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Mouse IgG2a Negative Control | MCA929 | F | 100 Tests |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Mouse IgG2a Negative Control | ||||||
References for WC1 antibody
-
Howard, C.J. et al. (1989) In vivo depletion of BoT4 (CD4) and of non-T4/T8 lymphocyte subsets in cattle with monoclonal antibodies.
Eur J Immunol. 19 (4): 757-64. -
Clevers, H. et al. (1990) Identification of a bovine surface antigen uniquely expressed on CD4-CD8- T cell receptor gamma/delta+ T lymphocytes.
Eur J Immunol. 20 (4): 809-17. -
Gutierrez, M. et al. (1999) The detection of CD2+, CD4+, CD8+, and WC1+ T lymphocytes, B cells and macrophages in fixed and paraffin embedded bovine tissue using a range of antigen recovery and signal amplification techniques.
Vet Immunol Immunopathol. 71 (3-4): 321-34. -
Brodersen, R. et al. (1998) Analysis of the immunological cross reactivities of 213 well characterized monoclonal antibodies with specificities against various leucocyte surface antigens of human and 11 animal species.
Vet Immunol Immunopathol. 64 (1): 1-13. -
Winkler, M.T. et al. (2000) Persistence and reactivation of bovine herpesvirus 1 in the tonsils of latently infected calves.
J Virol. 74 (11): 5337-46. -
Sanchez, J. et al. (2011) Microscopical and immunological features of tuberculoid granulomata and cavitary pulmonary tuberculosis in naturally infected goats.
J Comp Pathol. 145 (2-3): 107-17. -
Fulton, B.E. Jr. et al. (2006) Dissemination of bovine leukemia virus-infected cells from a newly infected sheep lymph node.
J Virol. 80: 7873-84. -
Glew, E.J. and Howard, C.J. (2001) Antigen-presenting cells from calves persistently infected with bovine viral diarrhoea virus, a member of the Flaviviridae, are not compromised in their ability to present viral antigen.
J Gen Virol. 82: 1677-85.
View The Latest Product References
-
Liebana, E. et al. (2007) Distribution and activation of T-lymphocyte subsets in tuberculous bovine lymph-node granulomas.
Vet Pathol. 44: 366-72. -
Lynch, E.M. et al. (2010) Effect of abrupt weaning at housing on leukocyte distribution, functional activity of neutrophils, and acute phase protein response of beef calves.
BMC Vet Res. 6: 39. -
Summers, C. et al. (2012) The distribution of immune cells in the lungs of classical and atypical ovine pulmonary adenocarcinoma.
Vet Immunol Immunopathol. 146: 1-7. -
Maślanka T et al. (2012) The presence of CD25 on bovine WC1+ γ/δ T cells is positively correlated with their production of IL-10 and TGF-β, but not IFN-γ.
Pol J Vet Sci. 15 (1): 11-20. -
Herzig, C.T. et al. (2010) Evolution of the CD163 family and its relationship to the bovine gamma delta T cell co-receptor WC1.
BMC Evol Biol. 10: 181. -
Romero-Palomo, F. et al. (2017) Immunopathologic Changes in the Thymus of Calves Pre-infected with BVDV and Challenged with BHV-1.
Transbound Emerg Dis. 64 (2): 574-84. -
Goh, S. et al. (2016) Identification of Theileria lestoquardi Antigens Recognized by CD8+ T Cells.
PLoS One. 11 (9): e0162571. -
Wattegedera, S.R. et al. (2017) Enhancing the toolbox to study IL-17A in cattle and sheep.
Vet Res. 48 (1): 20. -
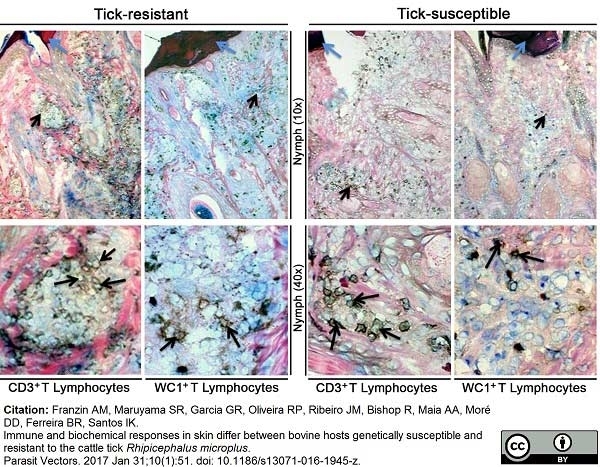
Franzin, A.M. et al. (2017) Immune and biochemical responses in skin differ between bovine hosts genetically susceptible and resistant to the cattle tick Rhipicephalus microplus.
Parasit Vectors. 10 (1): 51. -
Palomares, R.A. et al. (2015) Acute infection with bovine viral diarrhea virus of low or high virulence leads to depletion and redistribution of WC1(+) γδ T cells in lymphoid tissues of beef calves.
Vet Immunol Immunopathol. 167 (3-4): 190-5. -
Blumerman SL et al. (2007) Molecular cloning of bovine chemokine receptors and expression by WC1+ γδ T cells.
Dev Comp Immunol. 31 (1): 87-102. -
Hecker YP et al. (2013) Immune response and protection provided by live tachyzoites and native antigens from the NC-6 Argentina strain of Neospora caninum in pregnant heifers.
Vet Parasitol. 197 (3-4): 436-46. -
Martínez CM et al. (2005) Immunophenotypical characterization of lymphocyte subpopulations of the uterus of non-pregnant and pregnant goats.
Anat Histol Embryol. 34 (4): 240-6. -
Silva, A.P. et al. (2015) Encapsulated Brucella ovis Lacking a Putative ATP-Binding Cassette Transporter (ΔabcBA) Protects against Wild Type Brucella ovis in Rams.
PLoS One. 10 (8): e0136865. -
Albertsson, A.M. et al. (2018) γδ T Cells Contribute to Injury in the Developing Brain.
Am J Pathol. 188 (3): 757-67. -
Higgins, J.L. et al. (2018) Cell mediated immune response in goats after experimental challenge with the virulent Brucella melitensis strain 16M and the reduced virulence strain Rev. 1.
Vet Immunol Immunopathol. 202: 74-84. -
Baliu-piqué, M. et al. (2019) Age-related distribution and dynamics of T-cells in blood and lymphoid tissues of goats.
Dev Comp Immunol. 93: 1-10. -
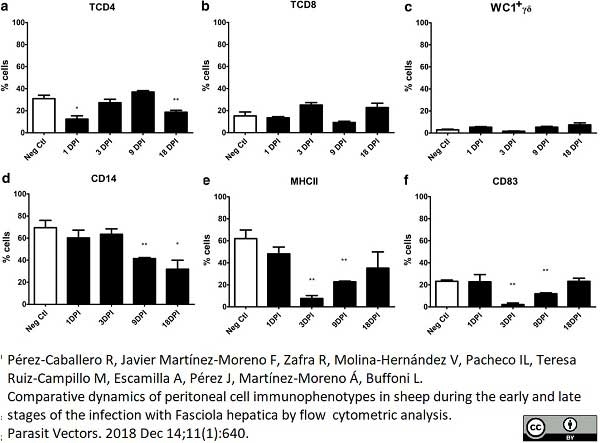
Pérez-caballero, R. et al. (2018) Comparative dynamics of peritoneal cell immunophenotypes in sheep during the early and late stages of the infection with Fasciola hepatica by flow cytometric analysis.
Parasit Vectors. 11 (1): 640. -
Gondaira, S. et al. (2020) Immunosuppression in Cows following Intramammary Infusion of Mycoplasma bovis.
Infect Immun. 88 (3)Feb 20 [Epub ahead of print]. -
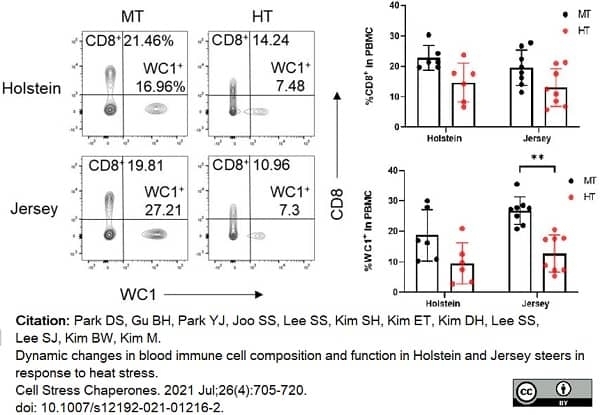
Park, D.S. et al. (2021) Dynamic changes in blood immune cell composition and function in Holstein and Jersey steers in response to heat stress.
Cell Stress Chaperones. 26 (4): 705-20. -
de Arujo, F.F. et al. (2019) Distinct immune response profile during Rhipicephalus (Boophilus) microplus. infestations of guzerat dairy herd according to the maternal lineage ancestry (mitochondrial DNA).
Vet Parasitol. 273: 36-44. -
Zecconi, A. et al. (2018) Effects of herd and physiological status on variation of 16 immunological and inflammatory parameters in dairy cows during drying off and the transition period.
J Dairy Res. 85 (2): 167-73. -
Colombatti, M.O. et al. (2021) Evaluation of a virulent strain of Mycobacterium avium subsp. Paratuberculosis used as a heat-killed vaccine.
Vaccine. 39 (51): 7401-12. -
Bidart, J. et al. (2020) A New Cage-Like Particle Adjuvant Enhances Protection of Foot-and-Mouth Disease Vaccine.
Front Vet Sci. 7: 396. -
Kolar, Q.K. et al. (2020) Anatomical distribution of respiratory tract leukocyte cell subsets in neonatal calves.
Vet Immunol Immunopathol. 227: 110090. -
Elsayed, M.S.A.E. et al. (2022) Real-time PCR using atpE, conventional PCR targeting different regions of difference, and flow cytometry for confirmation of Mycobacterium bovis. in buffaloes and cattle from the Delta area of Egypt.
BMC Microbiol. 22 (1): 154. -
Andrés, S. et al. (2024) Essential oil supplementation in milk replacers: short- and long-term impacts on feed efficiency, the faecal microbiota and the plasma metabolome in dairy calves.
J Dev Orig Health Dis. : 1-11.
- UniProt
- P30205
- Entrez Gene
- CD163L1
- GO Terms
- GO:0016020 membrane
- GO:0005576 extracellular region
- GO:0005044 scavenger receptor activity
MCA838GA
If you cannot find the batch/lot you are looking for please contact our technical support team for assistance.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Bovine ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up