CD31 antibody | CO.3E1D4
Mouse anti Sheep CD31
- Product Type
- Monoclonal Antibody
- Clone
- CO.3E1D4
- Isotype
- IgG2a
- Specificity
- CD31
| Mouse anti Sheep CD31 antibody, clone CO.3E1D4 recognizes ovine CD31, also known as PECAM-1. Ovine CD31 is predominantly expressed by peripheral blood platelets and a small percentage of lymphocytes. CD31 is also highly expressed by ovine endothelial cells. Mouse anti Sheep CD31 antibody, clone CO.3E1D4 is reported to inhibit homotypic leucocyte aggregation induced by anti CD43 antibodies (Pintado et al. 1995). |
- Target Species
- Sheep
- Species Cross-Reactivity
-
Target Species Cross Reactivity Goat Bovine - N.B. Antibody reactivity and working conditions may vary between species.
- Product Form
- Purified IgG - liquid
- Preparation
- Purified IgG prepared by affinity chromatography on Protein A from tissue culture supernatant
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
- 0.09% sodium azide (NaN3)
- Carrier Free
- Yes
- Immunogen
- Ovine leucocytes.
- Approx. Protein Concentrations
- IgG concentration 1.0 mg/ml
- Fusion Partners
- Spleen cells from immunised BALB/c mice were fused with cells of the SP2-0/Ag14 mouse myeloma cell line.
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Flow Cytometry | 1/10 | 1/25 | |
| Immunoprecipitation |
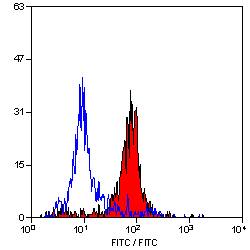
- Flow Cytometry
- Use 10μl of the suggested working dilution to label 1 x 106 cells in 100μl
Source Reference
-
Pintado. C. O. et al. (1995) A monoclonal antibody to an ovine gp130 molecule inhibits homotypic aggregation induced by anti CD43 monoclonal antibodies of ruminant leukocytes.
Immunol. Lett. 45: 81 - 85.
References for CD31 antibody
-
Brodersen, R. et al. (1998) Analysis of the immunological cross reactivities of 213 well characterized monoclonal antibodies with specificities against various leucocyte surface antigens of human and 11 animal species:
Vet. Immunol. Immunopathol. 64: 1-13. -
Newland, A. et al. (2004) Ovine dendritic cells transduced with an adenoviral CTLA4eEGFP fusion protein construct induce hyporesponsiveness to allostimulation.
Immunology. 113: 310-7. -
Summers, C. et al. (2005) An influx of macrophages is the predominant local immune response in ovine pulmonary adenocarcinoma.
Vet Immunol Immunopathol. 106 (3-4): 285-94. -
Zannettino, A.C. et al. (2010) Comparative assessment of the osteoconductive properties of different biomaterials in vivo seeded with human or ovine mesenchymal stem/stromal cells.
Tissue Eng Part A. 16 (12): 3579-87. -
De Visscher, G. et al. (2010) Selection of an immunohistochemical panel for cardiovascular research in sheep.
Appl Immunohistochem Mol Morphol. 18: 382-91. -
Filby, C.E. et al. (2010) Partial pulmonary embolization disrupts alveolarization in fetal sheep.
Respir Res. 11: 42. -
Boos, A.M. et al. (2011) Directly auto-transplanted mesenchymal stem cells induce bone formation in a ceramic bone substitute in an ectopic sheep model.
J Cell Mol Med. 15 (6): 1364-78. -
Berardinelli, P. et al. (2013) Role of amniotic fluid mesenchymal cells engineered on MgHA/collagen-based scaffold allotransplanted on an experimental animal study of sinus augmentation.
Clin Oral Investig. 17 (7): 1661-75.
View The Latest Product References
-
Barboni, B. et al. (2013) Synthetic bone substitute engineered with amniotic epithelial cells enhances bone regeneration after maxillary sinus augmentation.
PLoS One. 8 (5): e63256. -
Lasecka L et al. (2015) Antibodies to the core proteins of nairobi sheep disease virus/ganjam virus reveal details of the distribution of the proteins in infected cells and tissues.
PLoS One. 10 (4): e0124966. -
Iablonskii, P. et al. (2015) Tissue-engineered mitral valve: morphology and biomechanics †.
Interact Cardiovasc Thorac Surg. 20 (6): 712-9; discussion 719. -
Weigand, A. et al. (2017) Bone Tissue Engineering Under Xenogeneic-Free Conditions in a Large Animal Model as a Basis for Early Clinical Applicability.
Tissue Eng Part A. 23 (5-6): 208-22. -
Nielsen, E.Ø. et al. (2018) Optimizing Osteogenic Differentiation of Ovine Adipose-Derived Stem Cells by Osteogenic Induction Medium and FGFb, BMP2, or NELL1 In Vitro.
Stem Cells Int. 2018: 9781393. -
López-Fernández, A. et al. (2020) Effect of Allogeneic Cell-Based Tissue-Engineered Treatments in a Sheep Osteonecrosis Model.
Tissue Eng Part A. 26 (17-18): 993-1004.
- Synonyms
- PECAM-1
MCA1097GA
If you cannot find the batch/lot you are looking for please contact our technical support team for assistance.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Sheep ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up