CD31 antibody | TLD-3A12





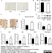






Mouse anti Rat CD31
- Product Type
- Monoclonal Antibody
- Clone
- TLD-3A12
- Isotype
- IgG1
- Specificity
- CD31
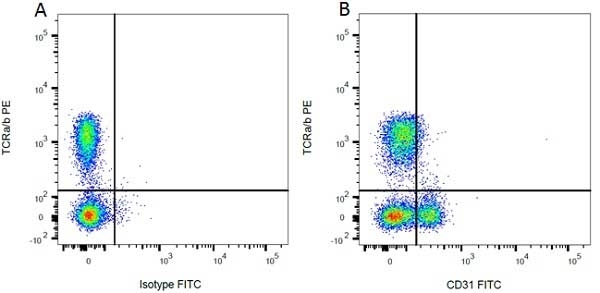
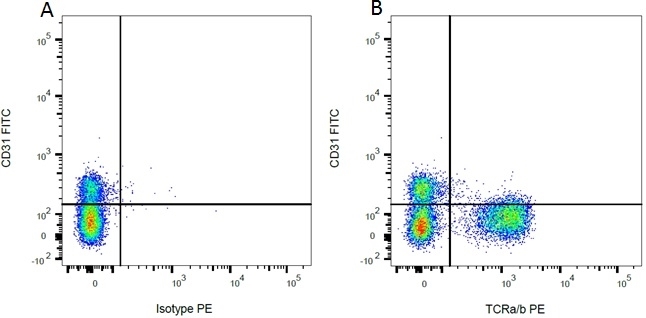
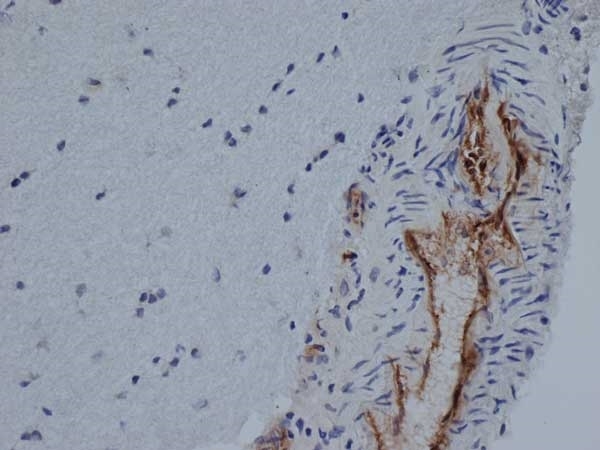
| Mouse anti Rat CD31 antibody, clone TLD-3A12 recognizes rat PECAM-1 (CD31), a 661 amino acid type 1 transmembrane protein expressed primarily on endothelial cells, platelets and leucocytes. Clone TLD-3A12 has been shown to partially block the proliferative response of antigen-specific CD4+ T cells to antigen-presenting cells and relevant antigen (Stevenson, K.S. et al.2009). Mouse anti Rat CD31 antibody, clone TLD-3A12 is suitable for use in IHC on formalin-fixed paraffin-embedded sections pre-treated with 0.2M boric acid, pH7.0. (Wilson et al. 2007). Mouse anti Rat CD31, clone TLD-3A12 has been shown to be cross-reactive with endothelial cells derived from rhesus macaque (Maclean et al. 2001) |
- Target Species
- Rat
- Species Cross-Reactivity
-
Target Species Cross Reactivity Rhesus Monkey Pig - N.B. Antibody reactivity and working conditions may vary between species.
- Product Form
- Purified IgG - liquid
- Preparation
- Purified IgG prepared by affinity chromatography on Protein A from tissue culture supernatant
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
- 0.09% sodium azide (NaN3)
- Carrier Free
- Yes
- Immunogen
- Activated, Lewis rat derived microglial cells.
- Approx. Protein Concentrations
- IgG concentration 1.0 mg/ml
- Fusion Partners
- Spleen cells from immunized BALB/c mouse were fused with cells of the mouse SP2 myeloma cell line.
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| ELISA | |||
| Flow Cytometry | 1/10 | 1/100 | |
| Immunofluorescence | |||
| Immunohistology - Frozen | 1/10 | 1/100 | |
| Western Blotting |
- Flow Cytometry
- Use 10μl of the suggested working dilution to label 106 cells in 100μl
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Mouse IgG1 Negative Control | MCA1209 | F | 0.1 mg |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Mouse IgG1 Negative Control | ||||||
Source Reference
-
Flaris, N.A. et al. (1993) Characterization of microglia and macrophages in the central nervous system of rats: definition of the differential expression of molecules using standard and novel monoclonal antibodies in normal CNS and in four models of parenchymal reaction.
Glia. 7 (1): 34-40.
References for CD31 antibody
-
Williams, K.C. et al. (1996) PECAM-1 (CD31) expression in the central nervous system and its role in experimental allergic encephalomyelitis in the rat.
J Neurosci Res. 45 (6): 747-57. -
Graham, M.J. et al. (1998) In vivo distribution and metabolism of a phosphorothioate oligonucleotide within rat liver after intravenous administration.
J Pharmacol Exp Ther. 286: 447-58. -
MacLean, A.G. et al. (2001) Rhesus macaque brain microvessel endothelial cells behave in a manner phenotypically distinct from umbilical vein endothelial cells.
J Neuroimmunol. 118: 223-32. -
Frye, C.A. & Patrick, C.W. Jr (2002) Isolation and culture of rat microvascular endothelial cells.
In Vitro Cell Dev Biol Anim. 38 (4): 208-12. -
Nakao, A. et al. (2003) Carbon monoxide inhalation protects rat intestinal grafts from ischemia/reperfusion injury.
Am J Pathol. 163: 1587-98. -
Seegers, H.C. et al. (2003) Enhancement of angiogenesis by endogenous substance P release and neurokinin-1 receptors during neurogenic inflammation.
J Pharmacol Exp Ther. 306: 8-12. -
Haywood, L. et al. (2003) Inflammation and angiogenesis in osteoarthritis.
Arthritis Rheum. 48: 2173-7. -
Wilson, E. et al. (2007) An evaluation of the immunohistochemistry benefits of boric acid antigen retrieval on rat decalcified joint tissues.
J Immunol Methods. 322: 137-42.
View The Latest Product References
-
Ohnishi, T. et al. (2007) Comparison of endothelial cell proliferation in normal liver and adipose tissue in B6C3F1 mice, F344 rats, and humans.
Toxicol Pathol. 35: 904-9. -
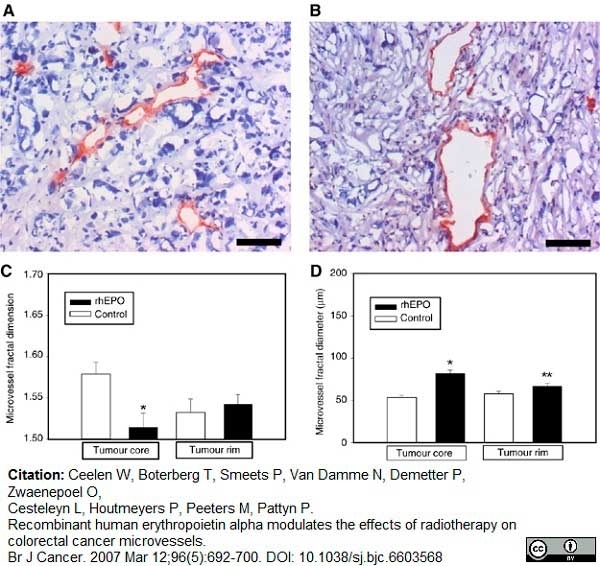
Ceelen, W. et al. (2007) Recombinant human erythropoietin alpha modulates the effects of radiotherapy on colorectal cancer microvessels.
Br J Cancer. 96: 692-700. -
Fujimoto, K.L. et al. (2007) An elastic, biodegradable cardiac patch induces contractile smooth muscle and improves cardiac remodeling and function in subacute myocardial infarction.
J Am Coll Cardiol. 49: 2292-300. -
Arkudas, A. et al. (2007) Fibrin gel-immobilized VEGF and bFGF efficiently stimulate angiogenesis in the AV loop model.
Mol Med. 13: 480-7. -
Schilte, M.N. et al. (2009) Long-term intervention with heparins in a rat model of peritoneal dialysis.
Perit Dial Int. 29: 26-35. -
Stevenson, K.S. et al. (2009) Isolation, characterization, and differentiation of thy1.1-sorted pancreatic adult progenitor cell populations.
Stem Cells Dev. 18 (10): 1389-98. -
Pedram, M.S. et al. (2010) Transplantation of a combination of autologous neural differentiated and undifferentiated mesenchymal stem cells into injured spinal cord of rats.
Spinal Cord. 48 (6): 457-63. -
Kielian, T. and Hickey, W.F. (2010) Proinflammatory cytokine, chemokine, and cellular adhesion molecule expression during the acute phase of experimental brain abscess development.
Am J Pathol. 157: 647-58. -
Thebault, P. et al. (2010) The C-type lectin-like receptor CLEC-1, expressed by myeloid cells and endothelial cells, is up-regulated by immunoregulatory mediators and moderates T cell activation.
J Immunol. 183: 3099-108. -
Willis, C.L. et al. (2010) Protein kinase C activation modulates reversible increase in cortical blood-brain barrier permeability and tight junction protein expression during hypoxia and posthypoxic reoxygenation.
J Cereb Blood Flow Metab. 30: 1847-59. -
Lochhead, J.J. et al. (2010) Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation.
J Cereb Blood Flow Metab. 30: 1625-36. -
Teng, B.T. et al. (2011) Protective effect of caspase inhibition on compression-induced muscle damage.
J Physiol. 589: 3349-69. -
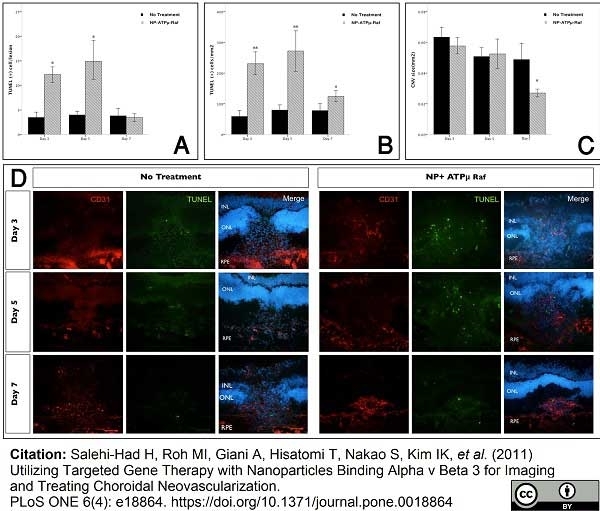
Salehi-Had, H. et al. (2011) Utilizing targeted gene therapy with nanoparticles binding alpha v beta 3 for imaging and treating choroidal neovascularization.
PLoS One. 6: e18864. -
Nakao, A. et al. (2011) Ex vivo carbon monoxide delivery inhibits intimal hyperplasia in arterialized vein grafts.
Cardiovasc Res. 89: 457-63. -
Sheu, J.J. et al. (2012) Combination of cilostazol and clopidogrel attenuates rat critical limb ischemia.
J Transl Med. 10: 164. -
Matsugami, H.et al. (2014) VEGF secretion by adipose tissue-derived regenerative cells is impaired under hyperglycemic conditions via glucose transporter activation and ROS increase.
Biomed Res. 35 (6): 397-405. -
Brandl, A. et al. (2014) A novel early precursor cell population from rat bone marrow promotes angiogenesis in vitro.
BMC Cell Biol. 15: 12. -
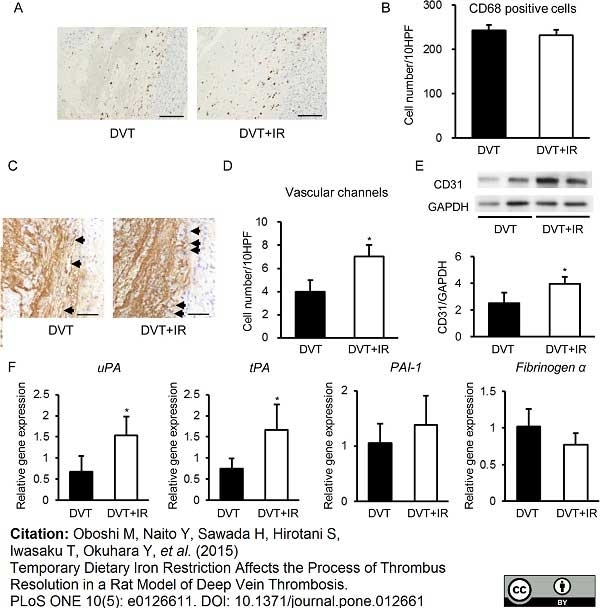
Oboshi, M. et al. (2015) Temporary dietary iron restriction affects the process of thrombus resolution in a rat model of deep vein thrombosis.
PLoS One. 10 (5): e0126611. -
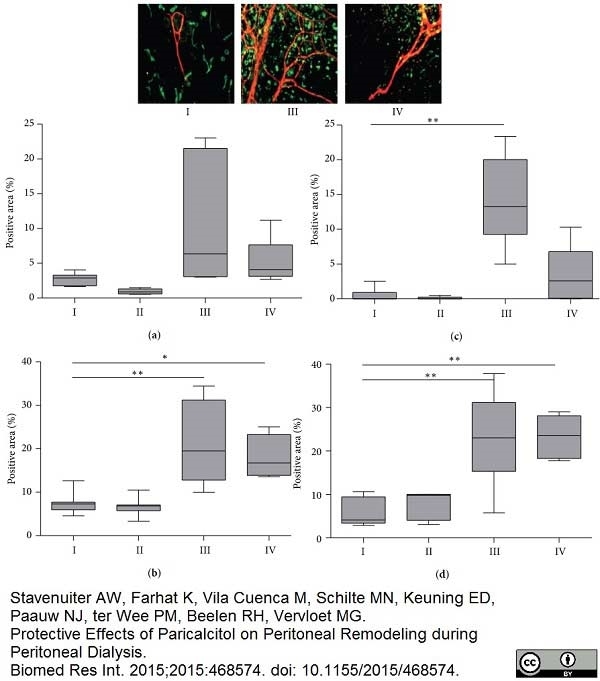
Stavenuiter, A.W. et al. (2015) Protective Effects of Paricalcitol on Peritoneal Remodeling during Peritoneal Dialysis.
Biomed Res Int. 2015: 468574. -
Naaijkens BA et al. (2015) Acute myocardial infarction does not affect functional characteristics of adipose-derived stem cells in rats, but reduces the number of stem cells in adipose tissue.
Cell Tissue Res. 362 (3): 623-32. -
Sun, C.K. et al. (2015) Mixed serum-deprived and normal adipose-derived mesenchymal stem cells against acute lung ischemia-reperfusion injury in rats.
Am J Transl Res. 7 (2): 209-31. -
Kakaiy, A. et al. (2015) Comparing protective effect of grape seed extract versus atorvastatin on endometriosis in rat model: Evidence for immunohistochemical and biochemical alterations.
Vet Res Forum. 6 (2): 101-10. -
Ikutomi, M. et al. (2015) Diverse contribution of bone marrow-derived late-outgrowth endothelial progenitor cells to vascular repair under pulmonary arterial hypertension and arterial neointimal formation.
J Mol Cell Cardiol. 86: 121-35. -
Wu, S.H. et al. (2015) Autologous adipose-derived stem cells attenuate muscular atrophy and protect spinal cord ventral horn motor neurons in an animal model of burn injury.
Cytotherapy. 17 (8): 1066-75. -
Jiang, Y. et al. (2015) SOD1 nanozyme salvages ischemic brain by locally protecting cerebral vasculature.
J Control Release. 213: 36-44. -
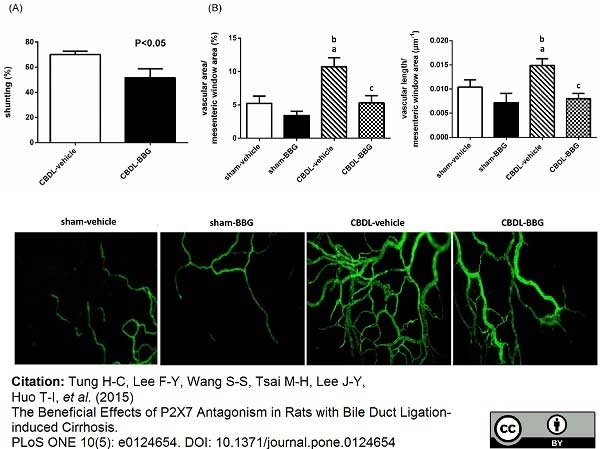
Tung, H.C. et al. (2015) The Beneficial Effects of P2X7 Antagonism in Rats with Bile Duct Ligation-induced Cirrhosis.
PLoS One. 10 (5): e0124654. -
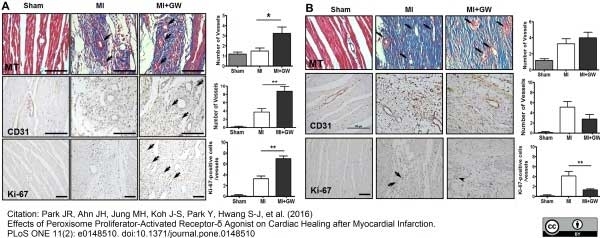
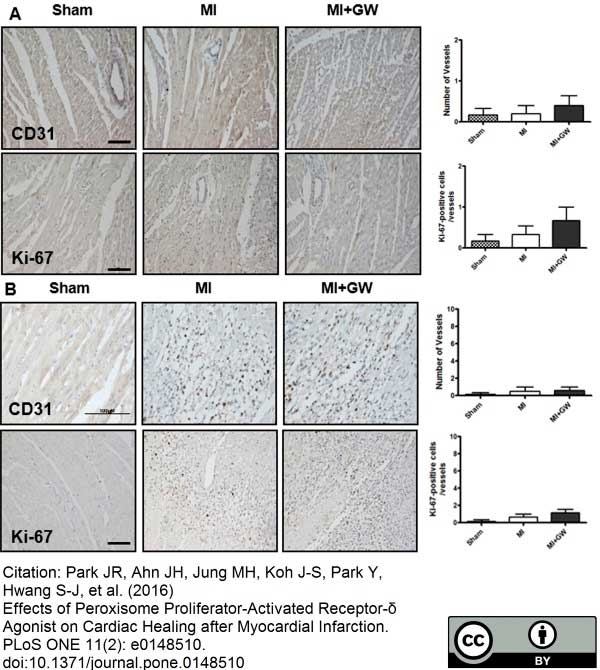
Park; J.R. et al. (2016) Effects of Peroxisome Proliferator-Activated Receptor-δ Agonist on Cardiac Healing after Myocardial Infarction.
PLoS One. 11 (2): e0148510. -
Lux, M. et al. (2016) In vitro maturation of large-scale cardiac patches based on a perfusable starter matrix by cyclic mechanical stimulation.
Acta Biomater. 30: 177-87. -
Ferrantelli, E. et al. (2016) The dipeptide alanyl-glutamine ameliorates peritoneal fibrosis and attenuates IL-17 dependent pathways during peritoneal dialysis.
Kidney Int. 89 (3): 625-35. -
Mirzaei, M. et al. (2017) Nanosilver particles increase follicular atresia: Correlation with oxidative stress and aromatization.
Environ Toxicol. 32 (10): 2244-55. -
Lim, S. et al. (2017) Attenuation of carotid neointimal formation after direct delivery of a recombinant adenovirus expressing glucagon-like peptide-1 in diabetic rats.
Cardiovasc Res. 113 (2): 183-94. -
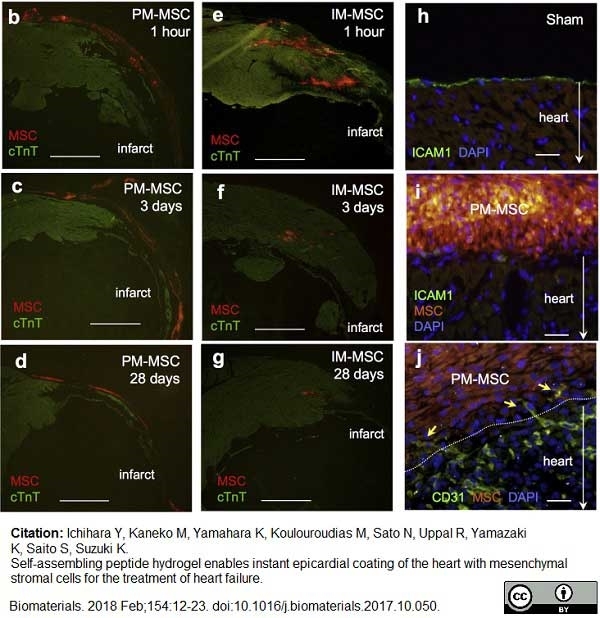
Ichihara, Y. et al. (2018) Self-assembling peptide hydrogel enables instant epicardial coating of the heart with mesenchymal stromal cells for the treatment of heart failure.
Biomaterials. 154: 12-23. -
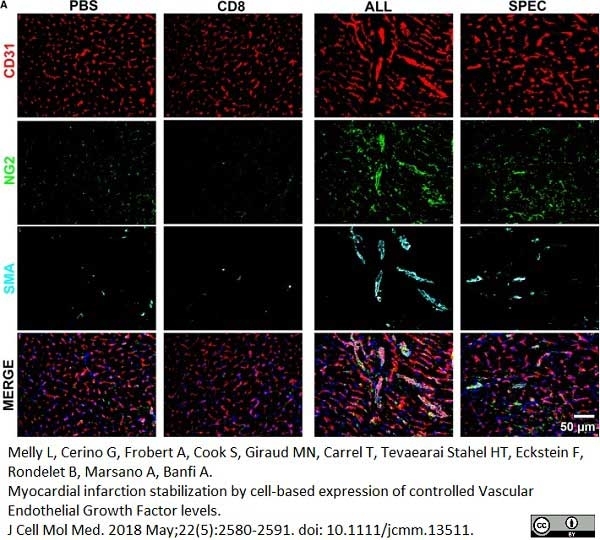
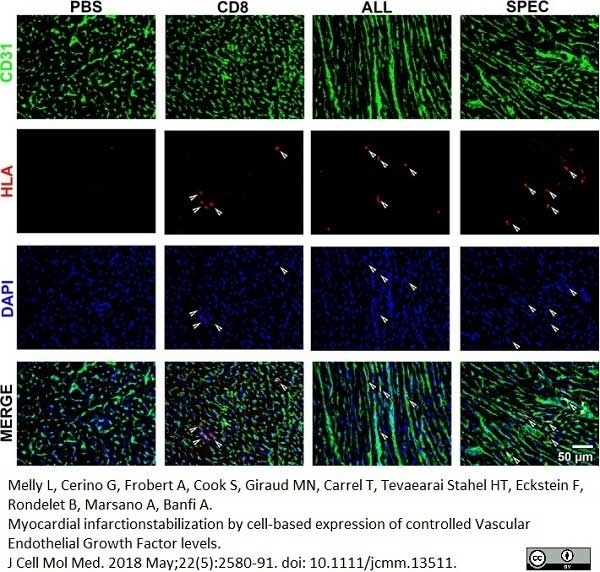
Melly, L. et al. (2018) Myocardial infarction stabilization by cell-based expression of controlled Vascular Endothelial Growth Factor levels.
J Cell Mol Med. 22 (5): 2580-91. -
Costa, B.P. et al. (2018) Intestinal Epithelial Stem Cells: Distinct Behavior After Surgical Injury and Teduglutide Administration.
J Invest Surg. 31 (3): 243-52. -
Sønstevold, T. et al. (2018) Hyperbaric oxygen treatment did not significantly affect radiation injury in the mandibular area of rats.
Oral Surg Oral Med Oral Pathol Oral Radiol. 125 (2): 112-9. -
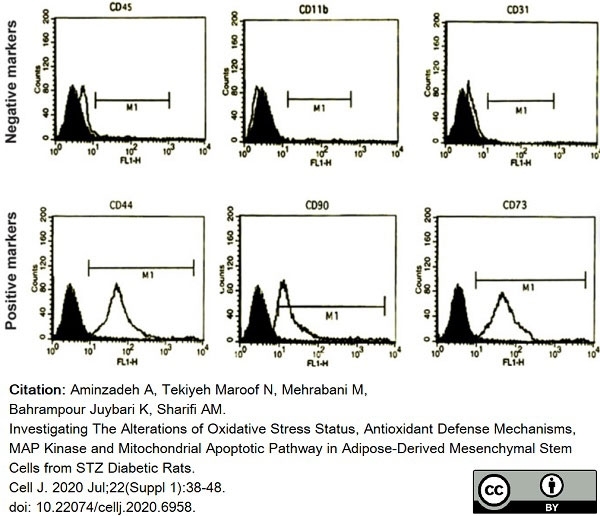
Aminzadeh, A. et al. (2020) Investigating The Alterations of Oxidative Stress Status, Antioxidant Defense Mechanisms, MAP Kinase and Mitochondrial Apoptotic Pathway in Adipose-Derived Mesenchymal Stem Cells from STZ Diabetic Rats.
Cell J. 22 (Suppl 1): 38-48. -
Kuriyama, T. et al. (2020) A novel rat model of inflammatory bowel disease developed using a device created with a 3D printer.
Regen Ther. 14: 1-10. -
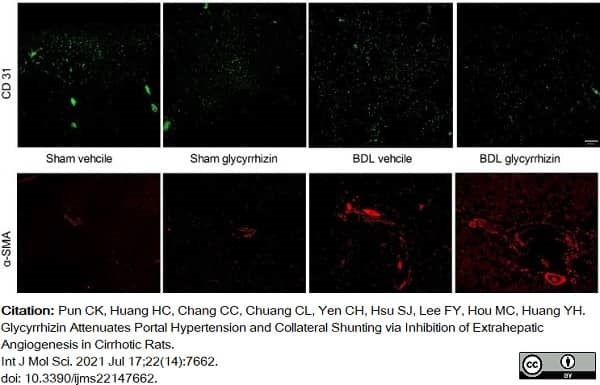
Pun, C.K. et al. (2021) Glycyrrhizin Attenuates Portal Hypertension and Collateral Shunting via Inhibition of Extrahepatic Angiogenesis in Cirrhotic Rats.
Int J Mol Sci. 22(14):7662. -
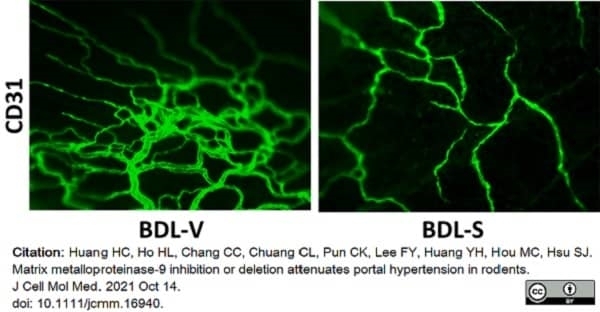
Huang, H.C. et al. (2021) Matrix metalloproteinase-9 inhibition or deletion attenuates portal hypertension in rodents.
J Cell Mol Med. 25 (21): 10073-87. -
Huang, H.C. et al. (2021) Microbiota transplants from feces or gut content attenuated portal hypertension and portosystemic collaterals in cirrhotic rats.
Clin Sci (Lond). 135 (24): 2709-2728. -
Cąkała-Jakimowicz, M. & Puzianowska-Kuznicka, M. (2022) Towards Understanding the Lymph Node Response to Skin Infection with Saprophytic Staphylococcus epidermidis..
Biomedicines. 10 (5): 1021. -
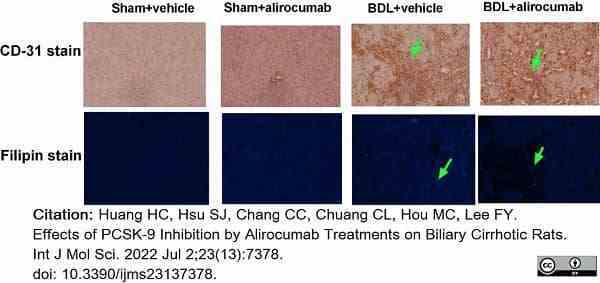
Huang, H.C. et al. (2022) Effects of PCSK-9 Inhibition by Alirocumab Treatments on Biliary Cirrhotic Rats.
Int J Mol Sci. 23 (13): 7378. -
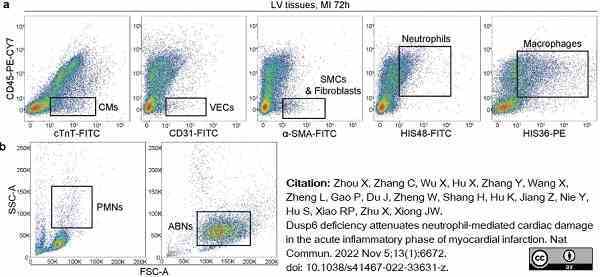
Zhou, X. et al. (2022) Dusp6 deficiency attenuates neutrophil-mediated cardiac damage in the acute inflammatory phase of myocardial infarction.
Nat Commun. 13 (1): 6672. -
Sun, Y. et al. (2023) Effects of acupuncture on angiogenesis-associated factor expression in ischemic brain tissue following cerebral infarction in rats
Acupuncture and Herbal Medicine. Jan [Epub ahead of print]. -
Zhang, Q. et al. (2023) Harnessing the synergy of perfusable muscle flap matrix and adipose-derived stem cells for prevascularization and macrophage polarization to reconstruct volumetric muscle loss.
Bioact Mater. 22: 588-614. -
Pun, C.K. et al. (2023) Dual angiotensin receptor and neprilysin inhibitor reduced portal pressure through peripheral vasodilatation and decreasing systemic arterial pressure in cirrhotic rats.
J Chin Med Assoc. 86 (9): 786-94. -
Wu, H.N. et al. (2023) Molecular Mechanism of Angiogenesis for Cerebral Infarction Rats by Acupuncture Intervention Based on Sonic Hedgehog Signaling Pathway.
Physiol Behav. Nov 28:114420 [Epub ahead of print]. -
Hsin, I.F. et al. (2018) Insulin reverses major portal hypertension-related derangements in rats with liver cirrhosis and diabetes.
Clin Sci (Lond). 132 (22): 2391-405. -
Wang, X. et al. (2024) Curcumol Attenuates Portal Hypertension and Collateral Shunting Via Inhibition of Extrahepatic Angiogenesis in Cirrhotic Rats.
Biochem Genet. Mar 04 [Epub ahead of print]. -
Huang, H.C. et al. (2022) Lycopene treatment improves intrahepatic fibrosis and attenuates pathological angiogenesis in biliary cirrhotic rats.
J Chin Med Assoc. 85 (4): 414-20.
- Synonyms
- PECAM-1
- RRID
- AB_322925
- UniProt
- Q3SWT0
- Entrez Gene
- Pecam1
- GO Terms
- GO:0005515 protein binding
- GO:0002687 positive regulation of leukocyte migration
- GO:0007155 cell adhesion
- GO:0016021 integral to membrane
- GO:0006928 cellular component movement
- GO:0009986 cell surface
- GO:0030054 cell junction
- GO:0034097 response to cytokine stimulus
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Rat ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up