CD14 antibody | MIL2








Mouse anti Pig CD14
- Product Type
- Monoclonal Antibody
- Clone
- MIL2
- Isotype
- IgG2b
- Specificity
- CD14
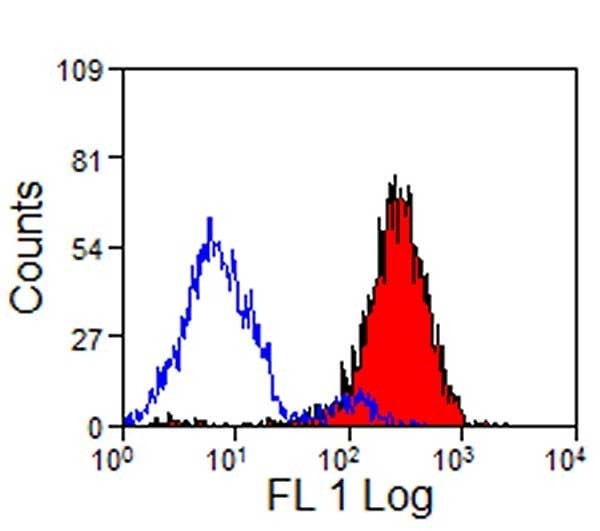
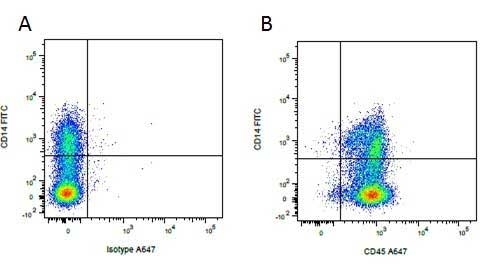
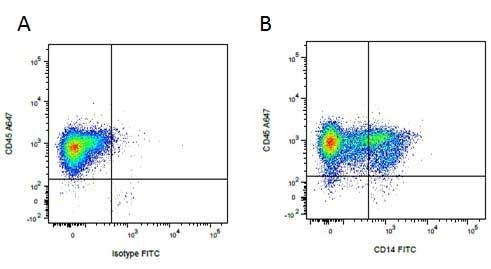
| Mouse anti Pig CD14, clone MIL2 recognizes porcine CD14. Clone MIL2 was clustered as porcine CD14 at the Third International Workshop on Swine Leukocyte Differentiation Antigens (Haverson et al. 2001) . Mouse anti Pig CD14, clone MIL2 immunoprecipitates a protein of ~50 kDa consistent with the expected apparent molecular weight of porcine CD14, and demonstrates the expected CD14 profile by dual labelling and competition assays. Further, pre-incubation of peripheral blood monocytes with MIL2 inhibits the binding of FITC labelled LPS, consistent with masking the CD14 LPS binding site (Thacker et al. 2001) . Mouse anti pig CD14, clone MIL2 demonstrates staining of both monocytes and neutrophils in peripheral blood by flow cytometry with a similar expression pattern to the anti human CD14 clone TüK4, lymphocytes and eosinophils are negative for MIL2 staining (Zelnickova et al. 2007). Cloning and characterization of porcine CD14 indicates a high degree of both functional and structural conservation when compared to CD14 from other mammalian species, the gene maps to chromosome 2 and is expressed on a wide range of tissues in a manner consistent with expression on myeloid cells. (Petersen et al. 2007, Sanz et al. 2007). |
- Target Species
- Pig
- Species Cross-Reactivity
-
Target Species Cross Reactivity Human - N.B. Antibody reactivity and working conditions may vary between species.
- Product Form
- Purified IgG - liquid
- Preparation
- Purified IgG prepared by affinity chromatography on Protein A from tissue culture supernatant
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
- 0.09% sodium azide (NaN3)
- Carrier Free
- Yes
- Immunogen
- Porcine peripheral blood lymphocytes.
- Approx. Protein Concentrations
- IgG concentration 1.0 mg/ml
- Fusion Partners
- Spleen cells from immunized Balb/c mice were fused with cells from the P2-X63-Ag.653 mouse myeloma.
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Flow Cytometry | 1/25 | 1/200 | |
| Immunofluorescence | |||
| Immunohistology - Frozen |
- Flow Cytometry
- Use 10μl of the suggested working dilution to label 106 cells in 100μl
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Mouse IgG2b Negative Control | MCA691 | F | 100 Tests |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Mouse IgG2b Negative Control | ||||||
Source Reference
-
Haverson, K. et al. (1994) Characterization of monoclonal antibodies specific for monocytes, macrophages and granulocytes from porcine peripheral blood and mucosal tissues.
J Immunol Methods. 170 (2): 233-45.
References for CD14 antibody
-
Hauet, T. et al. (2000) Trimetazidine reduces renal dysfunction by limiting the cold ischemia/reperfusion injury in autotransplanted pig kidneys.
J Am Soc Nephrol. 11: 138-48. -
Thacker, E. et al. (2001) Summary of workshop findings for porcine myelomonocytic markers.
Vet Immunol Immunopathol. 80 (1-2): 93-109. -
Thorgersen, E.B. et al. (2010) CD14 inhibition efficiently attenuates early inflammatory and hemostatic responses in Escherichia coli sepsis in pigs.
FASEB J. 24: 712-22. -
Goujon, J.M. et al. (2000) Influence of cold-storage conditions on renal function of autotransplanted large pig kidneys.
Kidney Int. 58: 838-50. -
Li, Y. et al. (2014) Identification of apoptotic cells in the thymus of piglets infected with highly pathogenic porcine reproductive and respiratory syndrome virus.
Virus Res. 189: 29-33. -
Summerfield, A. et al. (2003) Porcine peripheral blood dendritic cells and natural interferon-producing cells.
Immunology. 110: 440-9. -
Vanderheijden, N. et al. (2003) Involvement of sialoadhesin in entry of porcine reproductive and respiratory syndrome virus into porcine alveolar macrophages.
J Virol. 77: 8207-15. -
Barratt-Due, A. et al. (2011) Ornithodoros moubata Complement Inhibitor Is an Equally Effective C5 Inhibitor in Pigs and Humans.
J Immunol. 187: 4913-9.
View The Latest Product References
-
Hauet, T. et al. (2002) Polyethylene glycol reduces the inflammatory injury due to cold ischemia/reperfusion in autotransplanted pig kidneys.
Kidney Int. 62: 654-67. -
Kapetanovic, R. et al. (2012) Pig bone marrow-derived macrophages resemble human macrophages in their response to bacterial lipopolysaccharide.
J Immunol. 188: 3382-94. -
Thorgersen, E.B. et al. (2009) Inhibition of complement and CD14 attenuates the Escherichia coli-induced inflammatory response in porcine whole blood.
Infect Immun. 77: 725-32. -
Zelnickova, P. et al. (2007) Intracellular cytokine detection by flow cytometry in pigs: fixation, permeabilization and cell surface staining.
J Immunol Methods. 327: 18-29. -
Facci, M.R. et al. (2011) Stability of expression of reference genes in porcine peripheral blood mononuclear and dendritic cells.
Vet Immunol Immunopathol. 141: 11-5. -
Koutná, I. et al. (2012) Flow Cytometry Analysis of Intracellular Protein
In: Flow Cytometry - Recent Perspectives, Schmid, I. (Ed.), ISBN: 978-953-51. -
Facci, M.R. et al. (2010) A comparison between isolated blood dendritic cells and monocyte-derived dendritic cells in pigs.
Immunology. 129: 396-405. -
Schierack, P. et al. (2009) Effects of Bacillus cereus var. toyoi on immune parameters of pregnant sows.
Vet Immunol Immunopathol.127: 26-37. -
Lundeland, B. et al. (2011) Severe gunshot injuries in a porcine model: impact on central markers of innate immunity.
Acta Anaesthesiol Scand. 55: 28-34. -
Thorgersen, E.B. et al. (2008) Cyanobacterial LPS antagonist (CyP)-a novel and efficient inhibitor of Escherichia coli LPS-induced cytokine response in the pig.
Mol Immunol. 45: 3553-7. -
Schierack, P. et al. (2007) Bacillus cereus var. toyoi enhanced systemic immune response in piglets.
Vet Immunol Immunopathol. 118: 1-11. -
Ondrackova, P. et al. (2012) Interaction of porcine neutrophils with different strains of enterotoxigenic Escherichia coli.
Vet Microbiol. 160: 108-16. -
Ondrackova, P. et al. (2013) Phenotypic characterisation of the monocyte subpopulations in healthy adult pigs and Salmonella-infected piglets by seven-colour flow cytometry.
Res Vet Sci. 94 (2): 240-5. -
Vicenova, M. et al. (2014) Evaluation of in vitro and in vivo anti-inflammatory activity of biologically active phospholipids with anti-neoplastic potential in porcine model.
BMC Complement Altern Med. 14: 339. -
Alvarez, B. et al. (2015) Phenotypic and functional heterogeneity of CD169+ and CD163+ macrophages from porcine lymph nodes and spleen.
Dev Comp Immunol. 44: 44-9. -
Moffat, L. et al. (2014) Development and characterisation of monoclonal antibodies reactive with porcine CSF1R (CD115).
Dev Comp Immunol. 47 (1): 123-8. -
Kyrova K et al. (2014) The response of porcine monocyte derived macrophages and dendritic cells to Salmonella typhimurium and lipopolysaccharide.
BMC Vet Res. 10: 244. -
Nguyen, D.N. et al. (2016) Oral antibiotics increase blood neutrophil maturation and reduce bacteremia and necrotizing enterocolitis in the immediate postnatal period of preterm pigs.
Innate Immun. 22 (1): 51-62. -
Egge, K.H. et al. (2015) Organ inflammation in porcine Escherichia coli sepsis is markedly attenuated by combined inhibition of C5 and CD14.
Immunobiology. 220 (8): 999-1005. -
Liu J et al. (2016) The Role of Porcine Monocyte Derived Dendritic Cells (MoDC) in the Inflammation Storm Caused by Streptococcus suis Serotype 2 Infection.
PLoS One. 11 (3): e0151256. -
Singleton, H. et al. (2016) Establishing Porcine Monocyte-Derived Macrophage and Dendritic Cell Systems for Studying the Interaction with PRRSV-1.
Front Microbiol. 7: 832. -
Zemankova, N. et al. (2016) Bovine lactoferrin free of lipopolysaccharide can induce a proinflammatory response of macrophages.
BMC Vet Res. 12 (1): 251. -
Auray, G. et al. (2016) Characterization and Transcriptomic Analysis of Porcine Blood Conventional and Plasmacytoid Dendritic Cells Reveals Striking Species-Specific Differences.
J Immunol. 197 (12): 4791-806. -
Kavanová L et al. (2017) Concurrent infection with porcine reproductive and respiratory syndrome virus and Haemophilus parasuis in two types of porcine macrophages: apoptosis, production of ROS and formation of multinucleated giant cells.
Vet Res. 48 (1): 28. -
Bacou, E. et al. (2017) β2-adrenoreceptor stimulation dampens the LPS-induced M1 polarization in pig macrophages.
Dev Comp Immunol. 76: 169-76. -
Yang, G. et al. (2017) Characterizing porcine invariant natural killer T cells: A comparative study with NK cells and T cells.
Dev Comp Immunol. 76: 343-351. -
Uitterdijk, A. et al. (2017) Time course of VCAM-1 expression in reperfused myocardial infarction in swine and its relation to retention of intracoronary administered bone marrow-derived mononuclear cells.
PLoS One. 12 (6): e0178779. -
Sánchez, E.G. et al. (2017) Phenotyping and susceptibility of established porcine cells lines to African Swine Fever Virus infection and viral production.
Sci Rep. 7 (1): 10369. -
Fernández-Caballero, T. et al. (2018) Phenotypic and functional characterization of porcine bone marrow monocyte subsets.
Dev Comp Immunol. 81: 95-104. -
Sautter, C.A. et al. (2018) Phenotypic and functional modulations of porcine macrophages by interferons and interleukin-4.
Dev Comp Immunol. 84: 181-92. -
López, E. et al. (2019) Identification of very early inflammatory markers in a porcine myocardial infarction model.
BMC Vet Res. 15 (1): 91. -
Forner, R. et al. (2021) Distribution difference of colostrum-derived B and T cells subsets in gilts and sows.
PLoS One. 16 (5): e0249366. -
Skovdal, S.M. et al. (2019) Inhaled nebulized glatiramer acetate against Gram-negative bacteria is not associated with adverse pulmonary reactions in healthy, young adult female pigs.
PLoS One. 14 (10): e0223647. -
Vreman, S. et al. (2018) Neonatal porcine blood derived dendritic cell subsets show activation after TLR2 or TLR9 stimulation.
Dev Comp Immunol. 84: 361-70. -
Lau, C. et al. (2020) NHDL, a recombinant VL/VH hybrid antibody control for IgG2/4 antibodies.
MAbs. 12 (1): 1686319. -
Nielsen, O.L. et al. (2022) A porcine model of subcutaneous Staphylococcus aureus infection: a pilot study.
APMIS. 130 (7): 359-70. -
Melgoza-González, A.E. et al. (2022) Antigen Targeting of Porcine Skin DEC205+ Dendritic Cells
Vaccines. 10 (5): 684. -
Štěpánová, H. et al. (2022) Characterization of Porcine Monocyte-Derived Macrophages Cultured in Serum-Reduced Medium.
Biology (Basel). 11(10):1457. -
Monguió-Tortajada, M. et al. (2022) Acellular cardiac scaffolds enriched with MSC-derived extracellular vesicles limit ventricular remodelling and exert local and systemic immunomodulation in a myocardial infarction porcine model.
Theranostics. 12 (10): 4656-70. -
Bettin, L. et al. (2023) Co-stimulation by TLR7/8 ligand R848 modulates IFN-γ production of porcine γδ T cells in a microenvironment-dependent manner.
Dev Comp Immunol. 138: 104543. -
Haach, V. et al. (2023) A polyvalent virosomal influenza vaccine induces broad cellular and humoral immunity in pigs.
Virol J. 20 (1): 181. -
Li, J. et al. (2024) Single-cell transcriptomic analysis reveals transcriptional and cell subpopulation differences between human and pig immune cells.
Genes Genomics. 46 (3): 303-22. -
Álvarez, B. et al. (2023) Porcine Macrophage Markers and Populations: An Update.
Cells. 12 (16): 2103. -
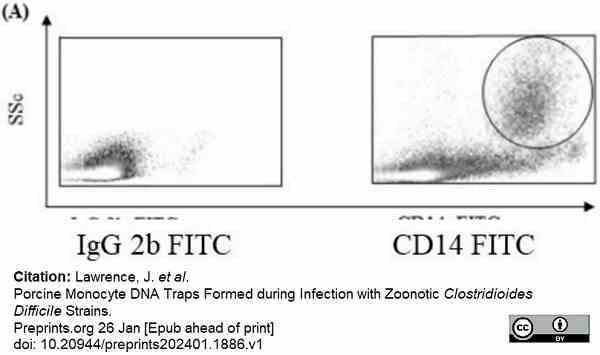
Lawrence, J. et al. (2024) Porcine Monocyte DNA Traps Formed during Infection with Pathogenic Clostridioides difficile Strains.
Pathogens. 13 (3): 228. -
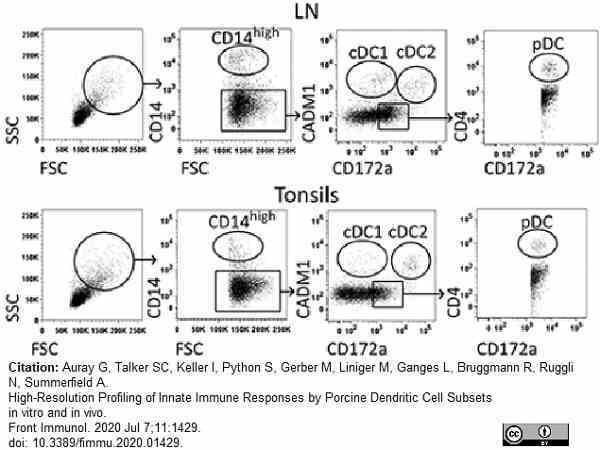
Auray, G. et al. (2020) High-Resolution Profiling of Innate Immune Responses by Porcine Dendritic Cell Subsets in vitro and in vivo.
Front Immunol. 11: 1429. -
Nieto-Pelegrín, E. et al. (2020) Porcine CLEC12B is expressed on alveolar macrophages and blood dendritic cells.
Dev Comp Immunol. 111: 103767. -
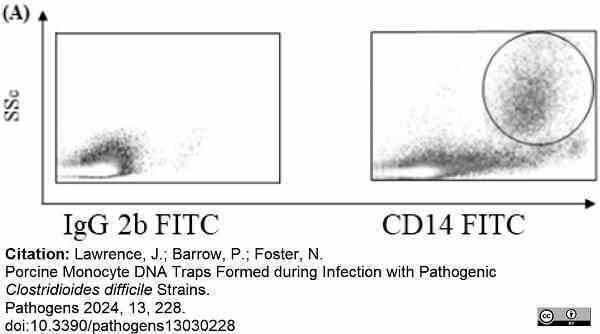
Lawrence, J. et al. (2024) Porcine Monocyte DNA Traps Formed during Infection with Pathogenic Clostridioides difficile Strains
Pathogens. 13 (3): 228. -
Jarosova, R. et al. (2022) Cytokine expression by CD163+ monocytes in healthy and Actinobacillus pleuropneumoniae-infected pigs.
Res Vet Sci. 152: 1-9. -
Waddell, L.A. et al. (2018) ADGRE1 (EMR1, F4/80) Is a Rapidly-Evolving Gene Expressed in Mammalian Monocyte-Macrophages.
Front Immunol. 9: 2246.
Further Reading
-
Piriou-Guzylack, L. (2008) Membrane markers of the immune cells in swine: an update.
Vet Res. 39: 54. -
Petersen, C.B. et al. (2007) Cloning, characterization and mapping of porcine CD14 reveals a high conservation of mammalian CD14 structure, expression and locus organization.
Dev Comp Immunol. 31: 729-37. -
Sanz, G. et al. (2007) Molecular cloning, chromosomal location, and expression analysis of porcine CD14.
Dev Comp Immunol. 31(7):738-47.
- UniProt
- A2SW51
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Pig ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up