CD68 antibody | FA-11
















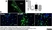






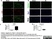


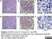
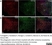
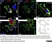

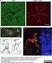







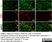





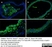







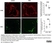
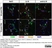















































Rat anti Mouse CD68
- Product Type
- Monoclonal Antibody
- Clone
- FA-11
- Isotype
- IgG2a
- Specificity
- CD68
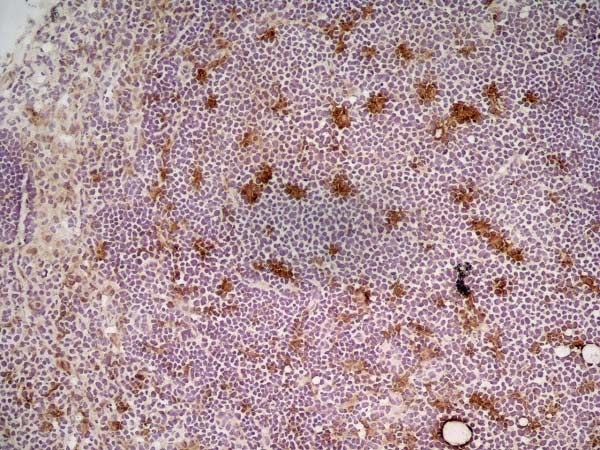
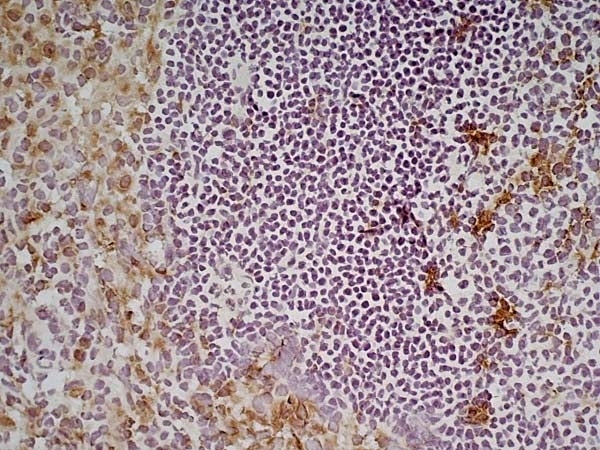
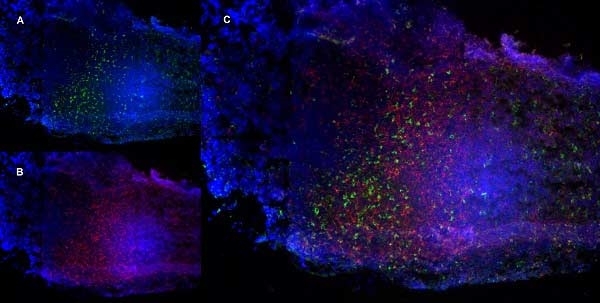
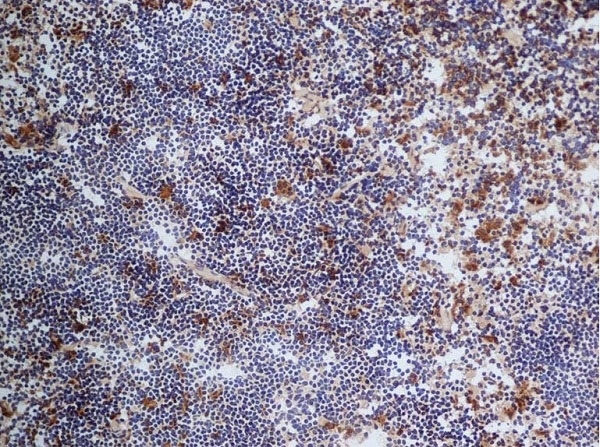
| Rat anti Mouse CD68 antibody, clone FA-11 recognizes mouse macrosialin, a heavily glycosylated transmembrane protein and murine homolog of human CD68, classified as a unique scavenger receptor (ScR) family member, due to the presence of a lysosome associated membrane protein (LAMP)-like domain. CD68 is considered a pan macrophage marker, predominantly expressed on the intracellular lysosomes of tissue macrophages/monocytes, including Kupffer cells, microglia, histiocytes and osteoclasts, and is expressed to a lesser extent by dendritic cells and peripheral blood granulocytes. CD68 is expressed by many tumor types including some B cell lymphomas, blastic NK lymphomas, melanomas, granulocytic (myeloid) sarcomas, hairy cell leukemias, and renal, urinary and pancreatic tumors, and can be used to demonstrate the presence/localization of macrophages. Rat anti mouse CD68 antibody, clone FA-11, has been used in many mouse models for the identification of CD68 in immunohistochemical assays, using both frozen and paraffin-embedded tissues (Masaki et al. 2003) and (Devey et al. 2009). Rat anti mouse CD68 antibody, clone FA-11 can be used in flow cytometry to detect intracellular CD68, following permeabilization, and can detect surface macrosialin at low levels in resident mouse peritoneal macrophages which can be enhanced with thioglycollate stimulation. |

Our CD68 (FA-11) Antibody has been referenced in >648 publications* *Based on June 2020 data from CiteAb's antibody search engine. |
- Target Species
- Mouse
- Product Form
- Purified IgG - liquid
- Preparation
- MCA1957T, MCA1957: Purified IgG prepared by affinity chromatography on Protein G from tissue culture supernatant.
- MCA1957GA: Purified IgG prepared by affinity chromatography on Protein G from tissue culture supernatant.
- Buffer Solution
- MCA1957T: Phosphate buffered saline.
- MCA1957GA, MCA1957: Phosphate buffered saline
- Preservative Stabilisers
- 0.09% sodium azide (NaN3)
- Carrier Free
- Yes
- Immunogen
- Purified Concanavalin A acceptor glycoprotein from P815 cell line.
- Approx. Protein Concentrations
- IgG concentration 1.0 mg/ml
- Regulatory
- MCA1957T: For research purposes only.
- MCA1957GA, MCA1957: For research purposes only
- Guarantee
- MCA1957T: 12 months from date of despatch.
- MCA1957GA, MCA1957: 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Flow Cytometry 1 | 1/50 | 1/100 | |
| Immunofluorescence | |||
| Immunohistology - Frozen | |||
| Immunohistology - Paraffin 2 | |||
| Immunoprecipitation | |||
| Western Blotting 3 |
- 1 Membrane permeabilization is required for this application. Bio-Rad recommends the use of Leucoperm (Product Code BUF09) for this purpose.
- 2This product may require antigen retrieval using heat treatment prior to staining of paraffin sections. Either sodium citrate buffer or Tris/EDTA buffer may be used for this purpose (Martin-Manso et al. 2008). Staining has also been achieved without antigen retrieval (Lu et al. 2010).
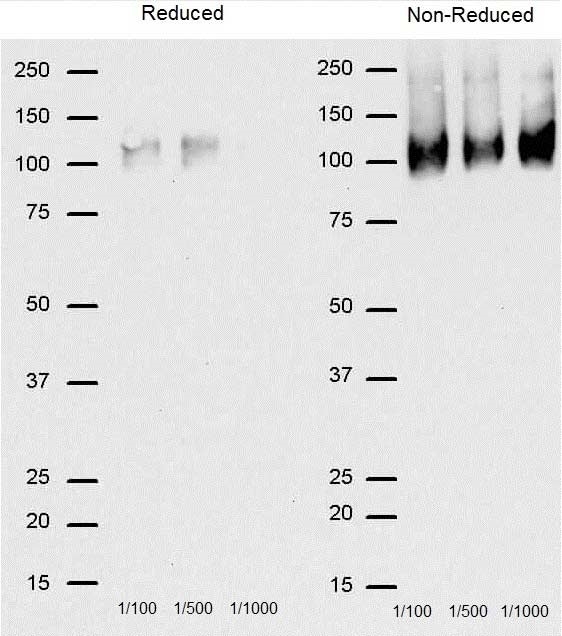
- 3 Non-reducing conditions recommended.
- Flow Cytometry
- Use 10μl of the suggested working dilution to label 106 cells in 100μl
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Rat IgG2a Negative Control | MCA1212 | E F | 1 ml |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Rat IgG2a Negative Control | ||||||
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Antigen Retrieval Buffer, pH8.0 | BUF025A | P | 500 ml | Log in | |||
| List Price | Your Price | ||||||
| Log in | |||||||
| Description | Antigen Retrieval Buffer, pH8.0 | ||||||
| Leucoperm | BUF09 | F | 50 Tests | Log in | |||
| List Price | Your Price | ||||||
| Log in | |||||||
| Description | Leucoperm | ||||||
Source Reference
-
Smith, M. and Koch, G. (1987) Differential expression of murine macrophage surface glycoprotein antigens in intracellular membranes.
J Cell Sci. 87:113-9.
References for CD68 antibody
-
Rabinowitz, S.S. & Gordon, S. (1991) Macrosialin, a macrophage-restricted membrane sialoprotein differentially glycosylated in response to inflammatory stimuli.
J Exp Med. 174 (4): 827-36. -
Ramprasad, M.P. et al. (1996) Cell surface expression of mouse macrosialin and human CD68 and their role as macrophage receptors for oxidized low density lipoprotein.
Proc Natl Acad Sci U S A. 93 (25): 14833-8. -
da Silva, R.P. & Gordon, S. (1999) Phagocytosis stimulates alternative glycosylation of macrosialin (mouse CD68), a macrophage-specific endosomal protein.
Biochem J. 338 ( Pt 3): 687-94. -
de Beer, M.C. et al. (2003) Lack of a direct role for macrosialin in oxidized LDL metabolism.
J Lipid Res. 44: 674-85. -
Masaki, T. et al. (2003) Heterogeneity of antigen expression explains controversy over glomerular macrophage accumulation in mouse glomerulonephritis.
Nephrol. Dial. Transplant 18:178-81. -
Schleicher, U. et al. (2005) Minute numbers of contaminant CD8+ T cells or CD11b+CD11c+ NK cells are the source of IFN-{gamma} in IL-12/IL-18-stimulated mouse macrophage populations.
Blood 105: 1319-1328. -
Echeverry, A. et al. (2007) Murine neonates are highly resistant to Yersinia enterocolitica following orogastric exposure.
Infect Immun. 75: 2234-43. -
Baran, C.P. et al. (2007) Important roles for macrophage colony-stimulating factor, CC chemokine ligand 2, and mononuclear phagocytes in the pathogenesis of pulmonary fibrosis.
Am J Respir Crit Care Med. 176: 78-89.
View The Latest Product References
-
Martin-Manso, G. et al. (2008) Thrombospondin 1 promotes tumor macrophage recruitment and enhances tumor cell cytotoxicity of differentiated U937 cells.
Cancer Res. 68: 7090-9. -
Sun, Y. et al. (2008) Temporal gene expression profiling reveals CEBPD as a candidate regulator of brain disease in prosaposin deficient mice.
BMC Neurosci. 9: 76. -
von Lukowicz, T. et al. (2008) PARP1 is required for adhesion molecule expression in atherogenesis.
Cardiovasc Res. 78 (1): 158-66. -
Sun, D. et al. (2008) Bone marrow-derived cell regulation of skeletal muscle regeneration.
FASEB J. 23: 382-95. -
Plüddemann, A. et al. (2009) The macrophage scavenger receptor A is host-protective in experimental meningococcal septicaemia.
PLoS Pathog. 5(2):e1000297. -
Bacci, M. et al. (2009) Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease
Am J Pathol. 175: 547-56. -
Hemmi, H. et al. (2009) A new triggering receptor expressed on myeloid cells (Trem) family member, Trem-like 4, binds to dead cells and is a DNAX activation protein 12-linked marker for subsets of mouse macrophages and dendritic cells.
J Immunol. 182: 1278-86. -
Devey, L. et al. (2009) Tissue-resident macrophages protect the liver from ischemia reperfusion injury via a heme oxygenase-1-dependent mechanism.
Mol Ther. 17: 65-72. -
Gräbner, R. et al. (2009) Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE-/- mice.
J Exp Med. 206: 233-48. -
Jayagopal, A. et al. (2009) Quantum dot mediated imaging of atherosclerosis.
Nanotechnology. 20: 165102. -
Leung, V.W. et al. (2009) Decay-accelerating factor suppresses complement C3 activation and retards atherosclerosis in low-density lipoprotein receptor-deficient mice.
Am J Pathol. 175: 1757-67. -
Yin, F. et al. (2010) Exaggerated inflammation, impaired host defense, and neuropathology in progranulin-deficient mice.
J Exp Med. 207: 117-28. -
Lu, W. et al. (2010) Photoacoustic imaging of living mouse brain vasculature using hollow gold nanospheres.
Biomaterials. 31: 2617-26. -
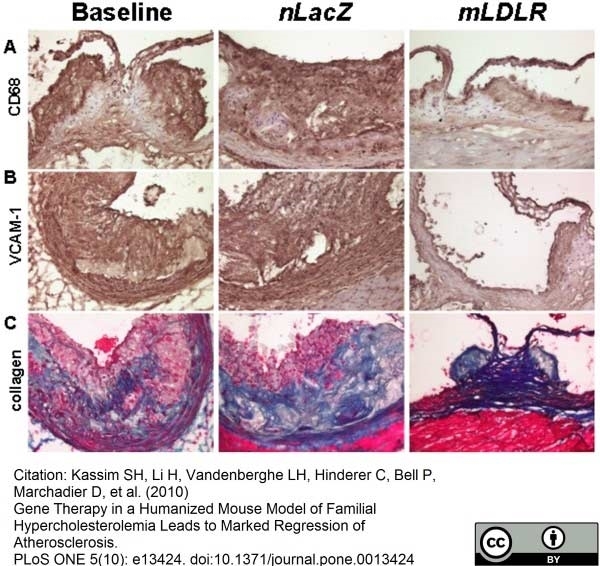
Kassim, S. et al. (2010) Gene therapy in a humanized mouse model of familial hypercholesterolemia leads to marked regression of atherosclerosis.
PloS ONE 5: e13424. -
Kassim, S.H. et al. (2010) Gene therapy in a humanized mouse model of familial hypercholesterolemia leads to marked regression of atherosclerosis.
PLoS One. 5(10):e13424. -
Doyle, K.P. et al. (2010) TGFβ signaling in the brain increases with aging and signals to astrocytes and innate immune cells in the weeks after stroke.
J Neuroinflammation. 7: 62. -
Chavele, K.M. et al. (2010) Mannose receptor interacts with Fc receptors and is critical for the development of crescentic glomerulonephritis in mice.
J Clin Invest. 120: 1469-78. -
Lebson, L. et al. (2010) Trafficking CD11b-positive blood cells deliver therapeutic genes to the brain of amyloid-depositing transgenic mice.
J Neurosci. 30: 9651-8. -
West, E.L. et al. (2010) Long-term survival of photoreceptors transplanted into the adult murine neural retina requires immune modulation.
Stem Cells. 28: 1997-2007. -
Frossard, J.L. et al. (2011) Role of CCL-2, CCR-2 and CCR-4 in cerulein-induced acute pancreatitis and pancreatitis-associated lung injury.
J Clin Pathol. 64: 387-93 -
Mortensen, M. et al. (2011) The autophagy protein Atg7 is essential for hematopoietic stem cell maintenance.
J Exp Med. 208: 455-67. -
Rahaman, S.O. et al. (2011) Vav family Rho guanine nucleotide exchange factors regulate CD36-mediated macrophage foam cell formation.
J Biol Chem. 286: 7010-7. -
Lopez, M.E. et al. (2011) Anatomically defined neuron-based rescue of neurodegenerative niemann-pick type C disorder.
J Neurosci. 31: 4367-78. -
Macauley, S.L. et al. (2011) The Role of Attenuated Astrocyte Activation in Infantile Neuronal Ceroid Lipofuscinosis
J. Neurosci 31: 15575-85. -
Daldrup-Link, H.E. et al. (2011) MR Imaging of Tumor Associated Macrophages with Clinically-Applicable Iron Oxide Nanoparticles.
Clin Cancer Res. 17: 5695-704. -
Song, L. et al. (2011) Deletion of the murine scavenger receptor CD68.
J Lipid Res. 52: 1542-50. -
Giannattasio, G. et al. (2011) The purinergic g protein-coupled receptor 6 inhibits effector T cell activation in allergic pulmonary inflammation.
J Immunol. 187: 1486-95. -
Papadeas, S.T. et al. (2011) Astrocytes carrying the superoxide dismutase 1 (SOD1G93A) mutation induce wild-type motor neuron degeneration in vivo.
Proc Natl Acad Sci U S A. 108: 17803-8. -
Lazarini, F. et al. (2012) Early Activation of Microglia Triggers Long-Lasting Impairment of Adult Neurogenesis in the Olfactory Bulb
J Neurosci 32: 3652-64 -
Kyaw, T. et al. (2012) Depletion of B2 but not B1a B cells in BAFF receptor-deficient ApoE mice attenuates atherosclerosis by potently ameliorating arterial inflammation.
PLoS One. 7: e29371. -
Akbarshahi, H. et al. (2012) Enrichment of Murine CD68(+)CCR2(+) and CD68(+)CD206(+) Lung Macrophages in Acute Pancreatitis-Associated Acute Lung Injury.
PLoS One. 7: e42654. -
Douglas, G. et al. (2012) Endothelial-Specific Nox2 Overexpression Increases Vascular Superoxide and Macrophage Recruitment in ApoE-/- mice.
Cardiovasc Res. 94: 20-9. -
Rojanathammanee, L. et al. (2013) Pomegranate polyphenols and extract inhibit nuclear factor of activated T-cell activity and microglial activation in vitro and in a transgenic mouse model of Alzheimer disease.
J Nutr. 143 (5): 597-605. -
Dormishian, M. et al. (2013) Prokineticin receptor-1 is a new regulator of endothelial insulin uptake and capillary formation to control insulin sensitivity and cardiovascular and kidney functions.
J Am Heart Assoc. 2 (5): e000411. -
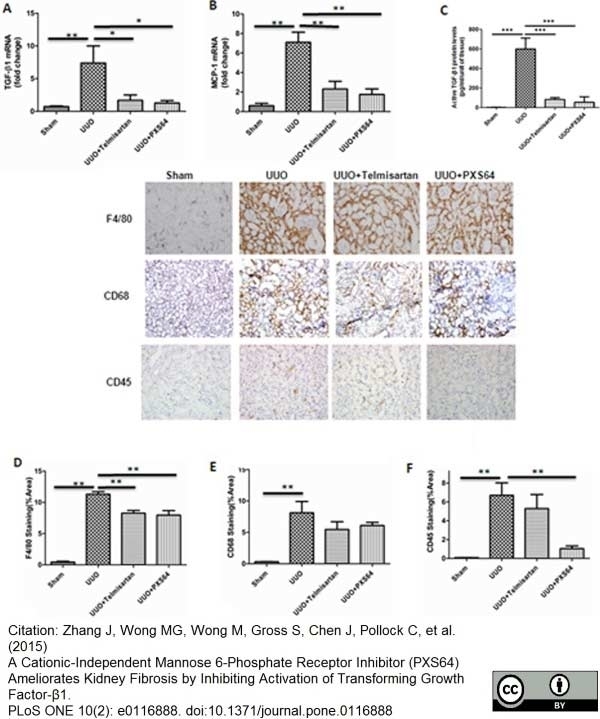
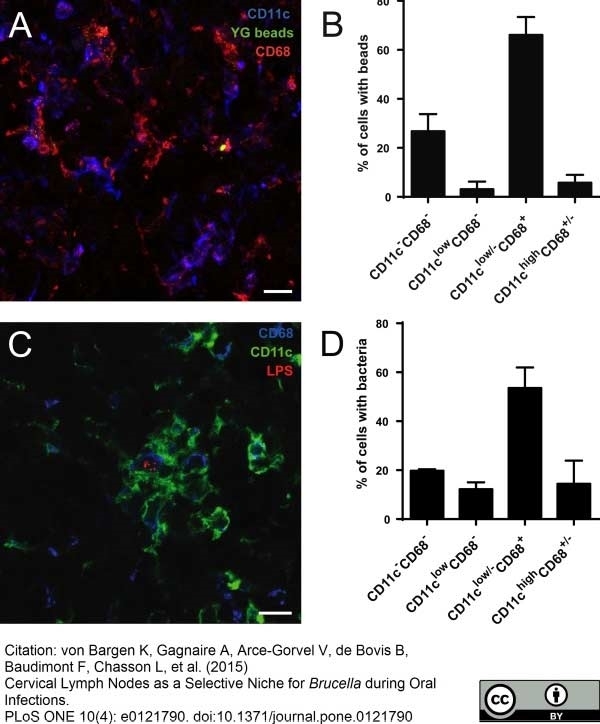
von Bargen, K. et al. (2014) Cervical Lymph Nodes as a Selective Niche for Brucella during Oral Infections.
PLoS One. 10 (4): e0121790. -
Choi, E.J. et al. (2014) Novel brain arteriovenous malformation mouse models for type 1 hereditary hemorrhagic telangiectasia.
PLoS One. 9(2): e88511. -
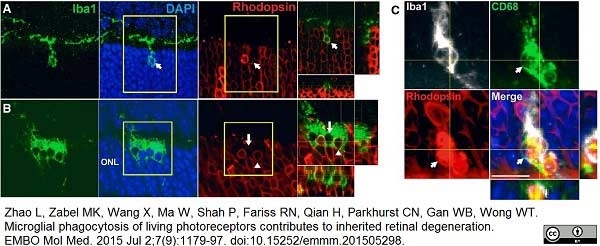
Nishikawa, K. et al. (2015) Resveratrol increases CD68⁺ Kupffer cells colocalized with adipose differentiation-related protein and ameliorates high-fat-diet-induced fatty liver in mice.
Mol Nutr Food Res. 59 (6): 1155-70. -
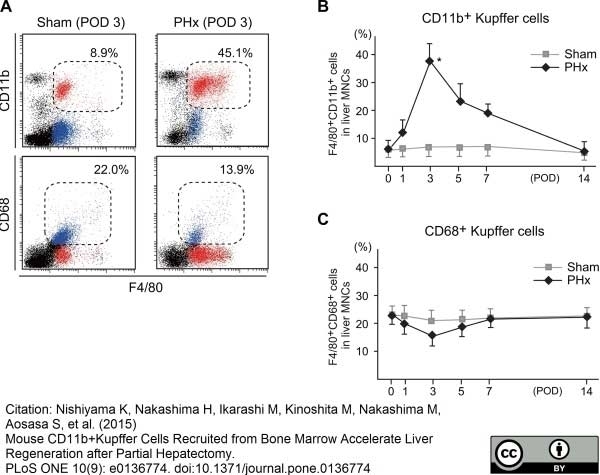
Nishiyama, K. et al. (2015) Mouse CD11b+ Kupffer Cells Recruited from Bone Marrow Accelerate Liver Regeneration after Partial Hepatectomy.
PLoS One. 10 (9): e0136774. -
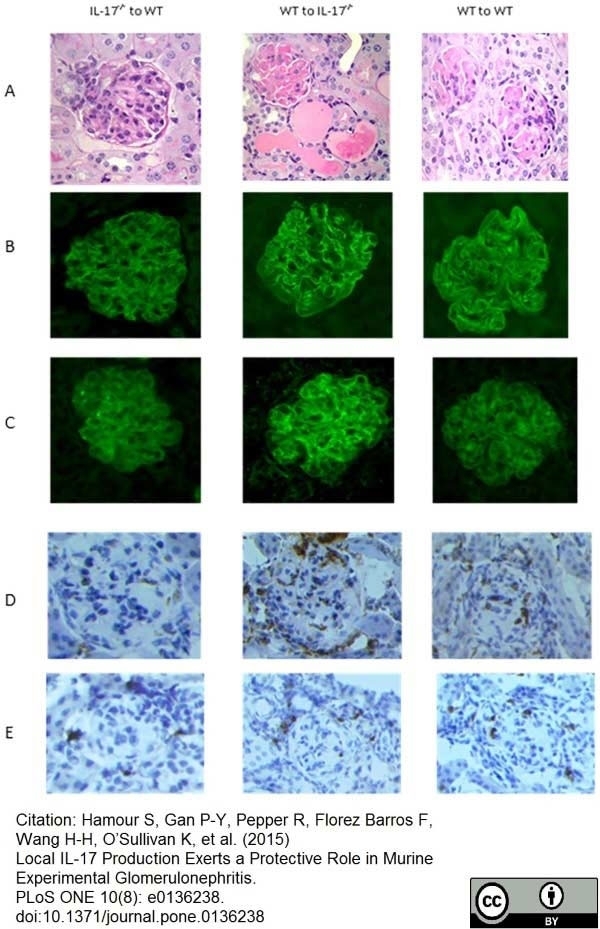
Hamour, S. et al. (2015) Local IL-17 Production Exerts a Protective Role in Murine Experimental Glomerulonephritis.
PLoS One. 10 (8): e0136238. -
Soto, I. et al. (2016) Meox2 haploinsufficiency increases neuronal cell loss in a mouse model of Alzheimer's disease.
Neurobiol Aging. 42: 50-60. -
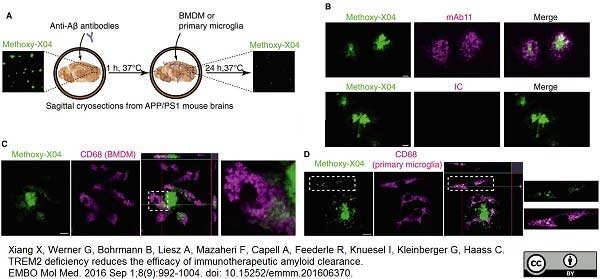
Xiang, X. et al. (2016) TREM2 deficiency reduces the efficacy of immunotherapeutic amyloid clearance.
EMBO Mol Med. 8 (9): 992-1004. -
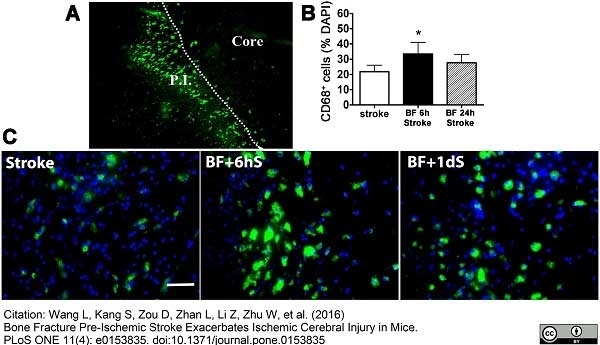
Wang L et al. (2016) Bone Fracture Pre-Ischemic Stroke Exacerbates Ischemic Cerebral Injury in Mice.
PLoS One. 11 (4): e0153835. -
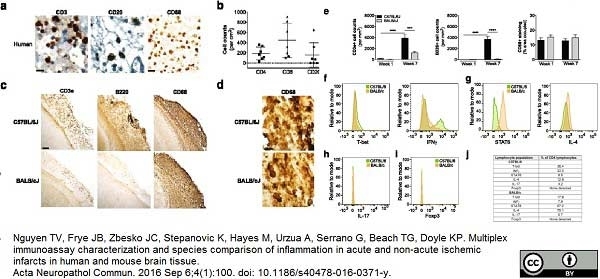
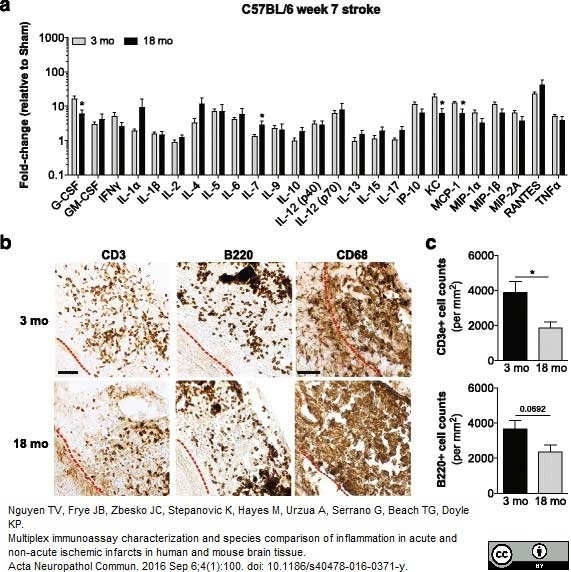
Nguyen, T.V. et al. (2016) Multiplex immunoassay characterization and species comparison of inflammation in acute and non-acute ischemic infarcts in human and mouse brain tissue.
Acta Neuropathol Commun. 4 (1): 100. -
Giraldo, J.A. et al. (2017) The impact of cell surface PEGylation and short-course immunotherapy on islet graft survival in an allogeneic murine model.
Acta Biomater. 49: 272-283. -
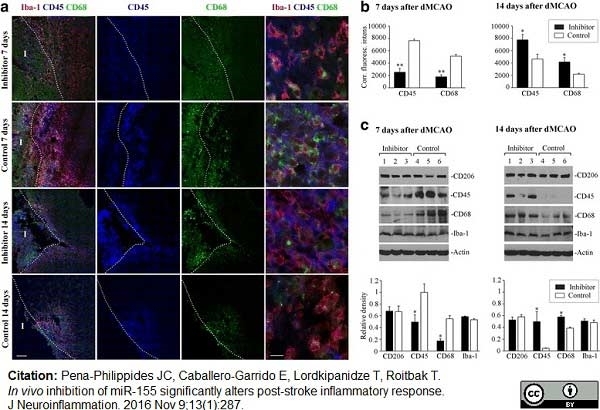
Pena-Philippides, J.C. et al. (2016) In vivo inhibition of miR-155 significantly alters post-stroke inflammatory response.
J Neuroinflammation. 13 (1): 287. -
Garofalo, S. et al. (2017) The Glycoside Oleandrin Reduces Glioma Growth with Direct and Indirect Effects on Tumor Cells.
J Neurosci. 37 (14): 3926-39. -
Nakaya, M. et al. (2017) Cardiac myofibroblast engulfment of dead cells facilitates recovery after myocardial infarction.
J Clin Invest. 127 (1): 383-401. -
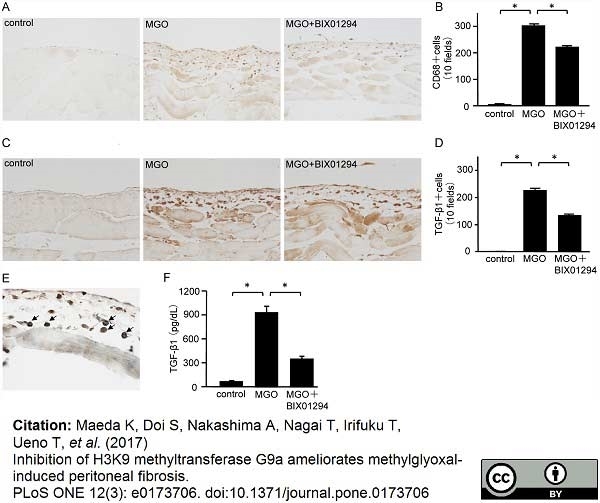
Maeda, K. et al. (2017) Inhibition of H3K9 methyltransferase G9a ameliorates methylglyoxal-induced peritoneal fibrosis.
PLoS One. 12 (3): e0173706. -
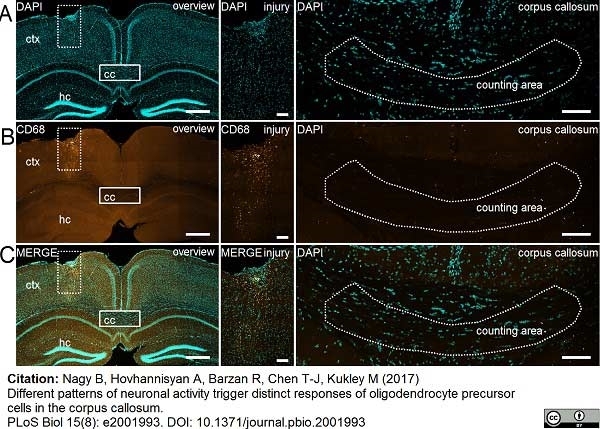
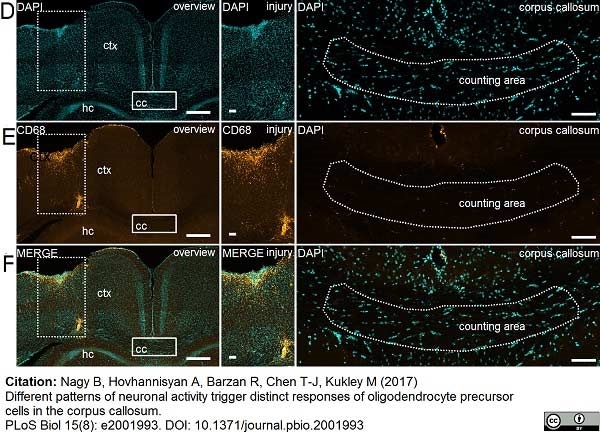
Nagy, B. et al. (2017) Different patterns of neuronal activity trigger distinct responses of oligodendrocyte precursor cells in the corpus callosum.
PLoS Biol. 15 (8): e2001993. -
Koh, A.J. et al. (2017) The skeletal impact of the chemotherapeutic agent etoposide.
Osteoporos Int. 28 (8): 2321-33. -
Menzies, R.I. et al. (2017) Hyperglycemia-induced Renal P2X7 Receptor Activation Enhances Diabetes-related Injury.
EBioMedicine. 19: 73-83. -
Takane, K. et al. (2017) Detrimental Effects of Centrally Administered Angiotensin II are Enhanced in a Mouse Model of Alzheimer Disease Independently of Blood Pressure.
J Am Heart Assoc. 6 (4): e004897. -
Xuan, H. et al. (2018) Inhibition or deletion of angiotensin II type 1 receptor suppresses elastase-induced experimental abdominal aortic aneurysms.
J Vasc Surg. 67 (2): 573-584.e2. -
Paiva, A. A. et al. (2017) Apolipoprotein CIII Overexpression-Induced Hypertriglyceridemia Increases Nonalcoholic Fatty Liver Disease in Association with Inflammation and Cell Death.
Oxidative Med Cellular Longev. 2017: 1-18. -
Rahman, K. et al. (2017) Inflammatory Ly6Chi monocytes and their conversion to M2 macrophages drive atherosclerosis regression.
J Clin Invest. 127 (8): 2904-15. -
Masuda, T. et al. (2017) Growth Factor Midkine Promotes T-Cell Activation through Nuclear Factor of Activated T Cells Signaling and Th1 Cell Differentiation in Lupus Nephritis.
Am J Pathol. 187 (4): 740-751. -
Chen, Y. et al. (2018) Progranulin associates with hexosaminidase A and ameliorates GM2 ganglioside accumulation and lysosomal storage in Tay-Sachs disease.
J Mol Med (Berl). 96 (12): 1359-73. -
Yuan, C. et al. (2018) Human Aldose Reductase Expression Prevents Atherosclerosis Regression in Diabetic Mice.
Diabetes. 67 (9): 1880-91. -
Kaji, N. et al. (2018) Disruption of the pacemaker activity of interstitial cells of Cajal via nitric oxide contributes to postoperative ileus.
Neurogastroenterol Motil. Mar 15 [Epub ahead of print]. -
Gökbuget, D. et al. (2018) The miRNA biogenesis pathway prevents inappropriate expression of injury response genes in developing and adult Schwann cells.
Glia. 66 (12): 2632-2644. -
Papaneophytou, C.P. et al. (2018) Regulatory role of oligodendrocyte gap junctions in inflammatory demyelination.
Glia. 66 (12): 2589-603. -
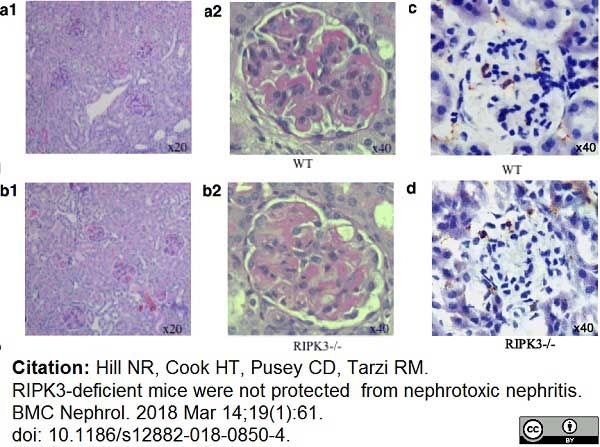
Hill, N.R. et al. (2018) RIPK3-deficient mice were not protected from nephrotoxic nephritis.
BMC Nephrol. 19 (1): 61. -
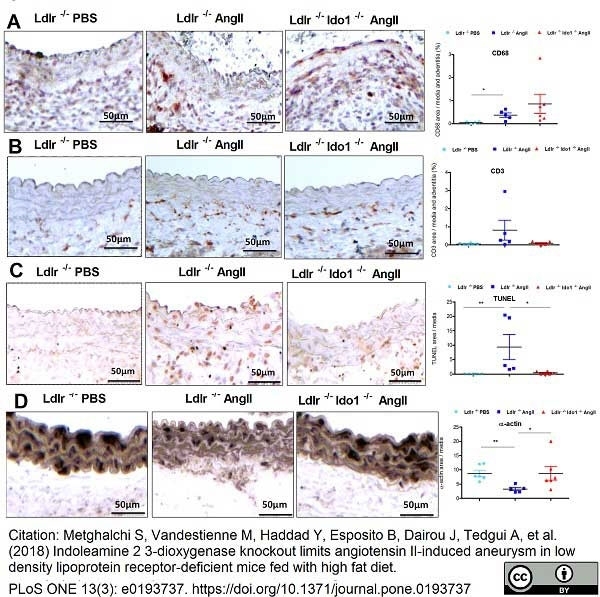
Metghalchi, S. et al. (2018) Indoleamine 2 3-dioxygenase knockout limits angiotensin II-induced aneurysm in low density lipoprotein receptor-deficient mice fed with high fat diet.
PLoS One. 13 (3): e0193737. -
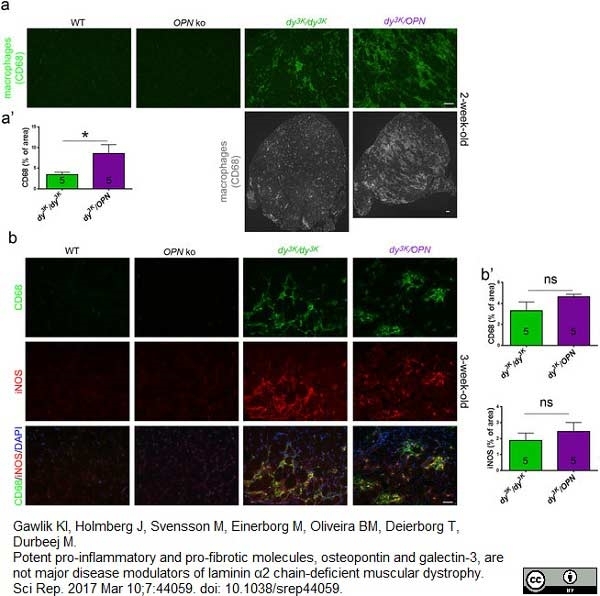
Welc, S.S. et al. (2019) Targeting a therapeutic LIF transgene to muscle via the immune system ameliorates muscular dystrophy.
Nat Commun. 10 (1): 2788. -
Säälik, P. et al. (2019) Peptide-guided nanoparticles for glioblastoma targeting.
J Control Release. 308: 109-18. -
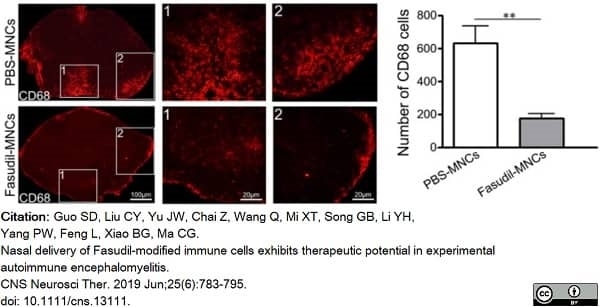
Guo, S.D. et al. (2019) Nasal delivery of Fasudil-modified immune cells exhibits therapeutic potential in experimental autoimmune encephalomyelitis.
CNS Neurosci Ther. 25 (6): 783-95. -
Zhang, Y. et al. (2019) Angiotensin II deteriorates advanced atherosclerosis by promoting MerTK cleavage and impairing efferocytosis through the AT1R/ROS/p38 MAPK/ADAM17 pathway.
Am J Physiol Cell Physiol. 317 (4): C776-C787. -
Wagner, M. et al. (2019) Clinical improvement and enhanced collateral vessel growth after xenogenic monocyte transplantation.
Am J Transl Res. 11 (7): 4063-76. -
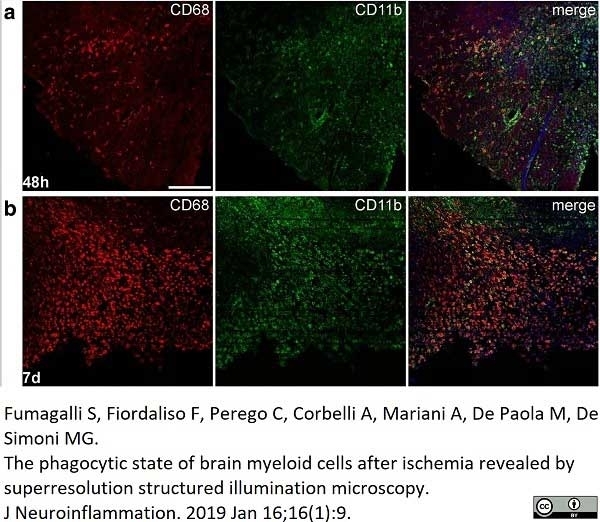
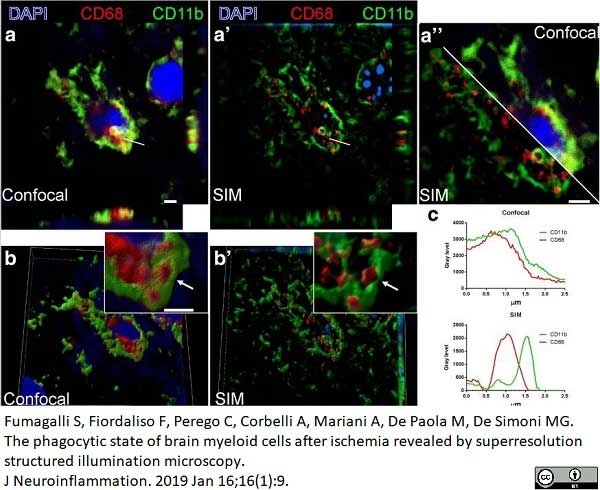
Fumagalli, S. et al. (2019) The phagocytic state of brain myeloid cells after ischemia revealed by superresolution structured illumination microscopy.
J Neuroinflammation. 16 (1): 9. -
Johnson, T.B. et al. (2019) Changes in motor behavior, neuropathology, and gut microbiota of a Batten disease mouse model following administration of acidified drinking water.
Sci Rep. 9 (1): 14962. -
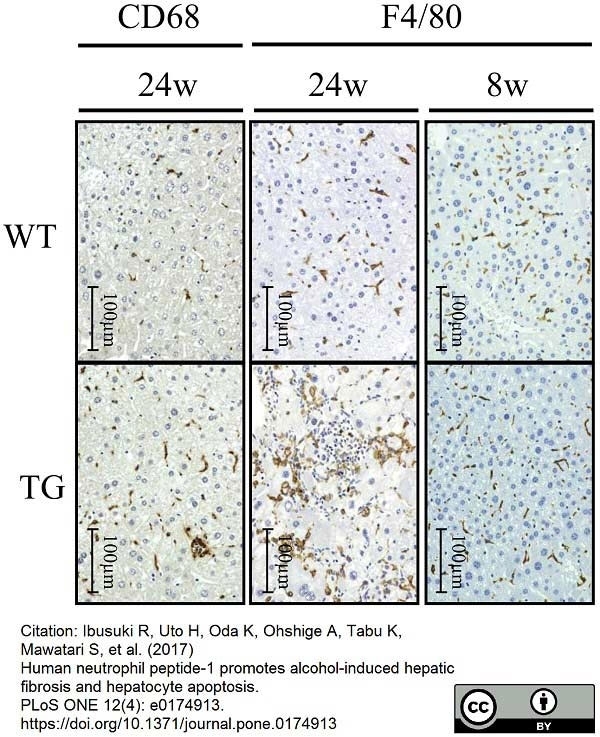
Ilyas, G. et al. (2019) Decreased Macrophage Autophagy Promotes Liver Injury and Inflammation from Alcohol.
Alcohol Clin Exp Res. 43 (7): 1403-13. -
El Gaamouch, F. et al. (2020) VGF-derived peptide TLQP-21 modulates microglial function through C3aR1 signaling pathways and reduces neuropathology in 5xFAD mice.
Mol Neurodegener. 15 (1): 4. -
Miteva, K. et al. (2020) Cardiotrophin-1 Deficiency Abrogates Atherosclerosis Progression.
Sci Rep. 10 (1): 5791. -
Galle-Treger, L. et al. (2020) Targeted invalidation of SR-B1 in macrophages reduces macrophage apoptosis and accelerates atherosclerosis.
Cardiovasc Res. 116 (3): 554-565. -
Yeo, K.P. et al. (2020) Efficient aortic lymphatic drainage is necessary for atherosclerosis regression induced by ezetimibe.
Sci Adv. 6 (50): eabc2697. -
Riester, K. et al. (2020) In vivo. characterization of functional states of cortical microglia during peripheral inflammation.
Brain Behav Immun. 87: 243-55. -
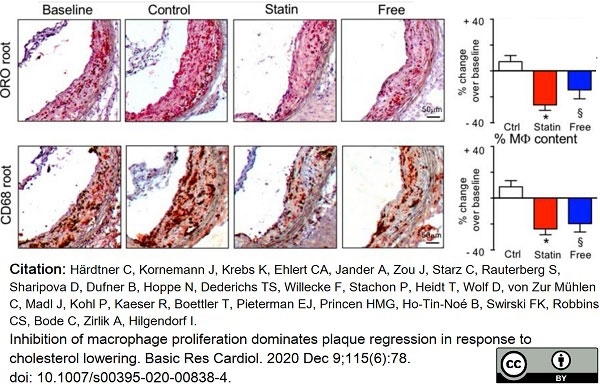
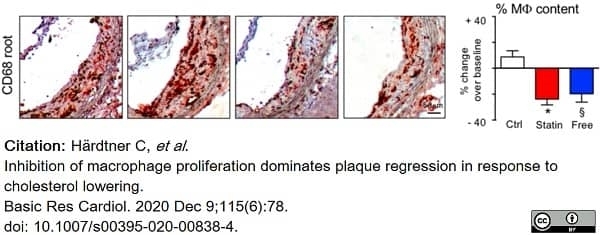
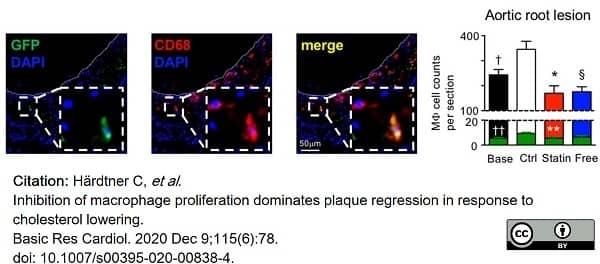
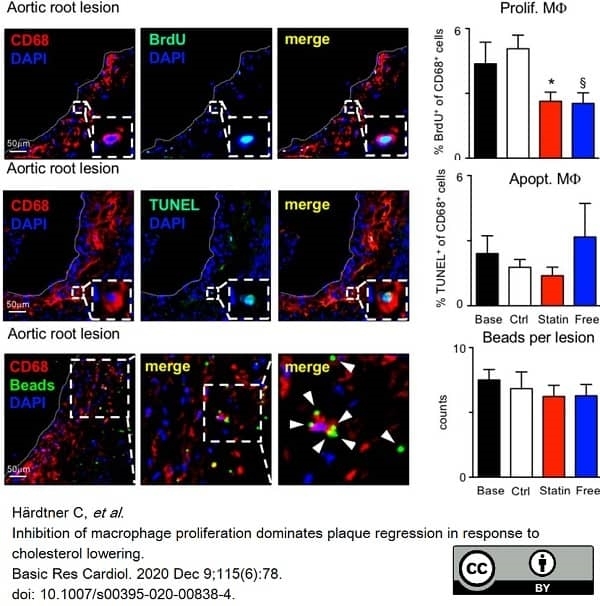
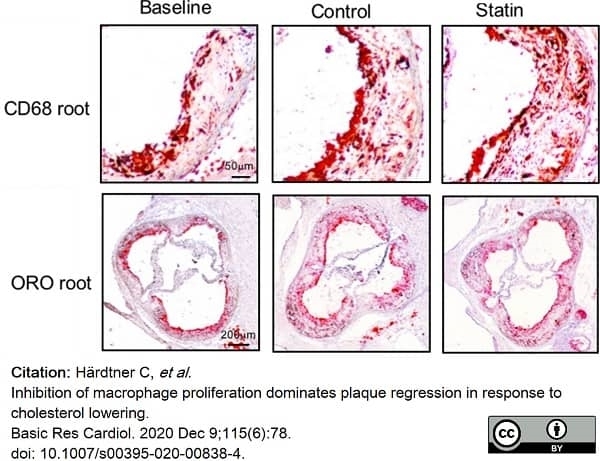
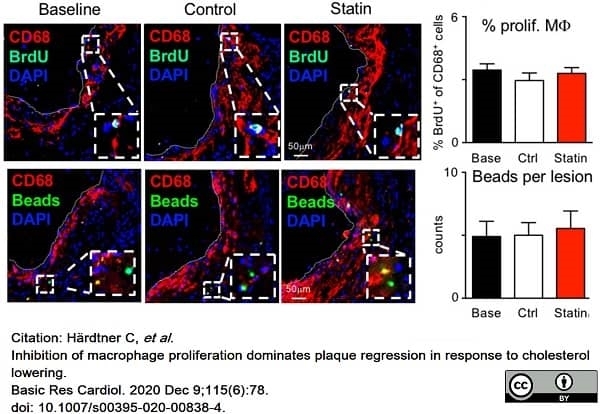
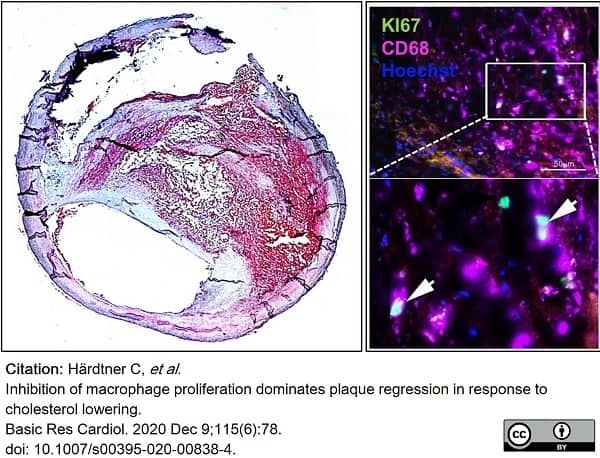
Härdtner, C. et al. (2020) Inhibition of macrophage proliferation dominates plaque regression in response to cholesterol lowering.
Basic Res Cardiol. 115 (6): 78. -
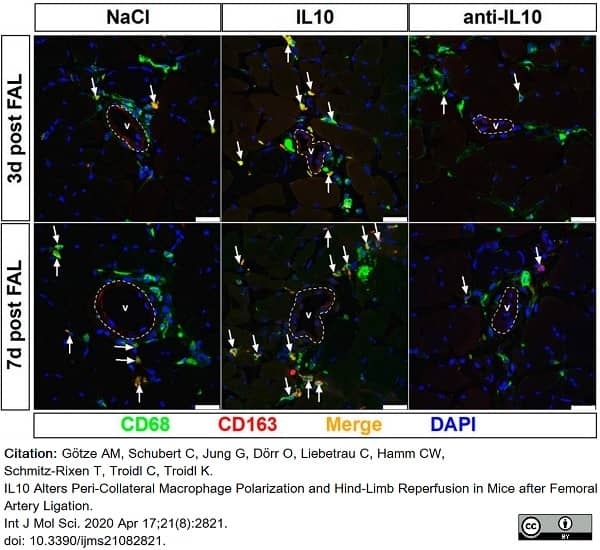
Götze, A.M. et al. (2020) IL10 Alters Peri-Collateral Macrophage Polarization and Hind-Limb Reperfusion in Mice after Femoral Artery Ligation.
Int J Mol Sci. 21 (8): 2821. -
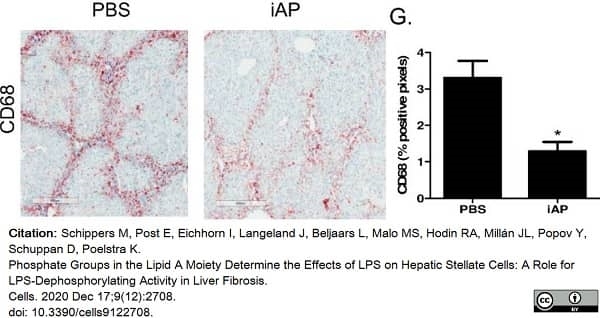
Schippers, M. et al. (2020) Phosphate Groups in the Lipid A Moiety Determine the Effects of LPS on Hepatic Stellate Cells: A Role for LPS-Dephosphorylating Activity in Liver Fibrosis.
Cells. 9 (12): 2708. -
Lim, S.L. et al. (2020) Genetic Ablation of Hematopoietic Cell Kinase Accelerates Alzheimer's Disease-Like Neuropathology in Tg2576 Mice.
Mol Neurobiol. 57 (5): 2447-60. -
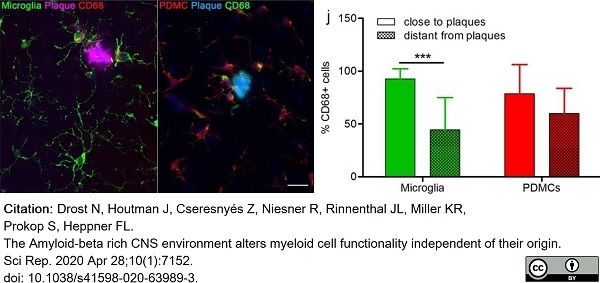
Drost, N. et al. (2020) The Amyloid-beta rich CNS environment alters myeloid cell functionality independent of their origin.
Sci Rep. 10 (1): 7152. -
Gratuze, M. et al.. (2020) Impact of TREM2R47H variant on tau pathology-induced gliosis and neurodegeneration
J Clin Invest130(9):4954-68. -
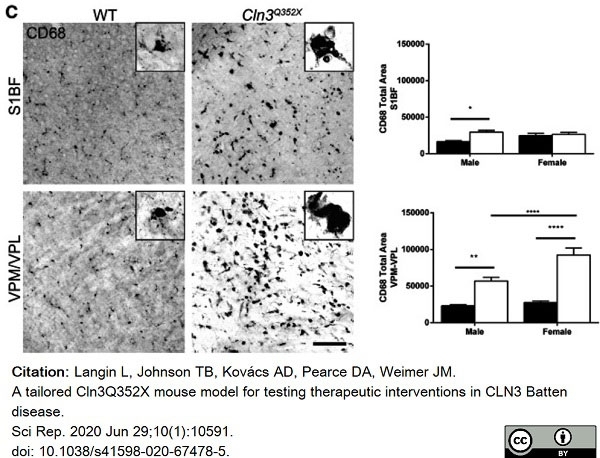
Langin, L. et al. (2020) A tailored Cln3Q352X mouse model for testing therapeutic interventions in CLN3 Batten disease.
Sci Rep. 10 (1): 10591. -
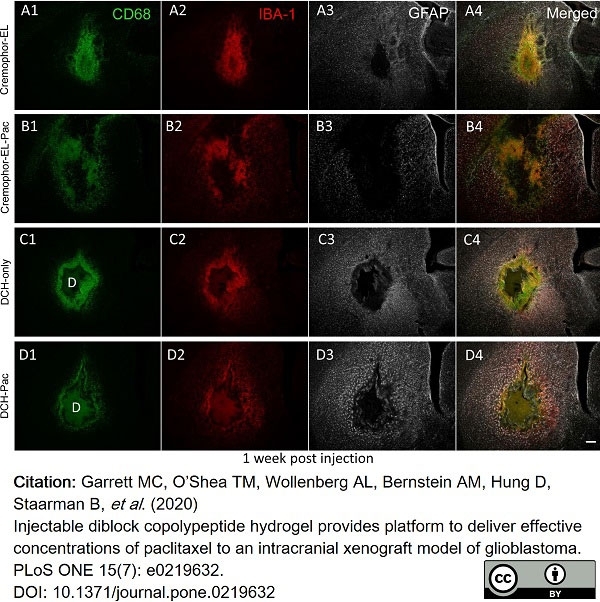
Garrett, M.C. et al. (2020) Injectable diblock copolypeptide hydrogel provides platform to deliver effective concentrations of paclitaxel to an intracranial xenograft model of glioblastoma.
PLoS One. 15 (7): e0219632. -
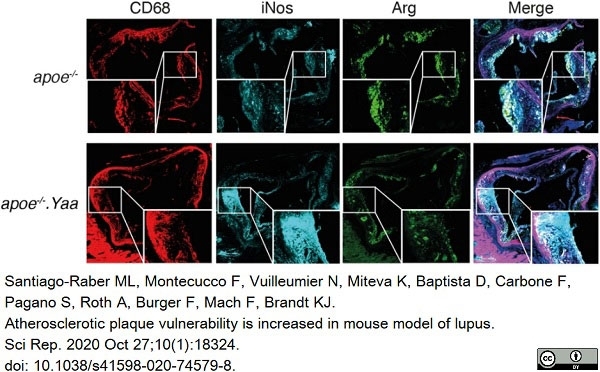
Santiago-Raber, M.L. et al. (2020) Atherosclerotic plaque vulnerability is increased in mouse model of lupus.
Sci Rep. 10 (1): 18324. -
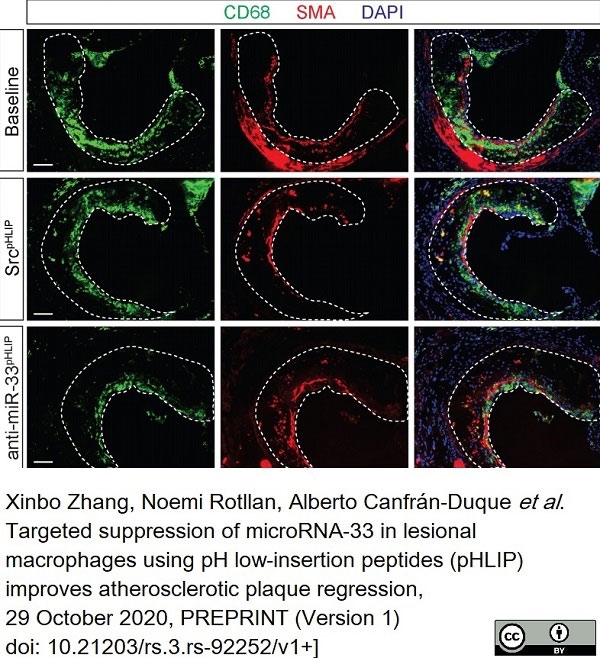
Zhang, X. et al. (2022) Targeted Suppression of miRNA-33 Using pHLIP Improves Atherosclerosis Regression.
Circ Res. 131 (1): 77-90. -
Hada, Y. et al. (2020) Inhibition of interleukin-6 signaling attenuates aortitis, left ventricular hypertrophy and arthritis in interleukin-1 receptor antagonist deficient mice.
Clin Sci (Lond). 134 (20): 2771-87. -
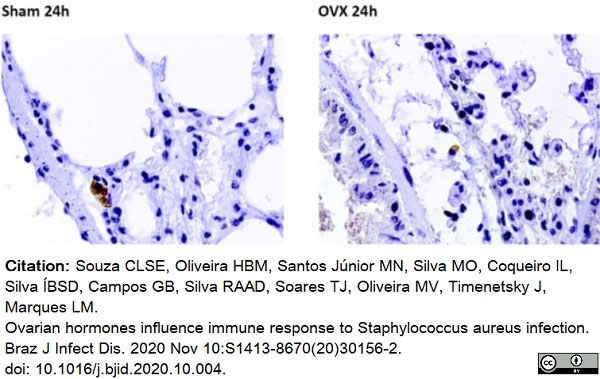
Souza, C.L.S.E. et al. (2020) Ovarian hormones influence immune response to Staphylococcus aureus infection.
Braz J Infect Dis. 24 (6): 534-44. -
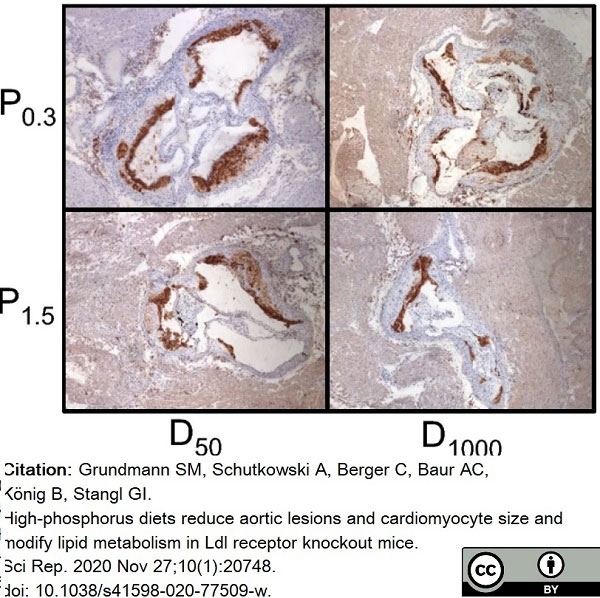
Grundmann, S.M. et al. (2020) High-phosphorus diets reduce aortic lesions and cardiomyocyte size and modify lipid metabolism in Ldl receptor knockout mice.
Sci Rep. 10 (1): 20748. -
Mia, M.M. et al. (2020) YAP/TAZ deficiency reprograms macrophage phenotype and improves infarct healing and cardiac function after myocardial infarction.
PLoS Biol. 18 (12): e3000941. -
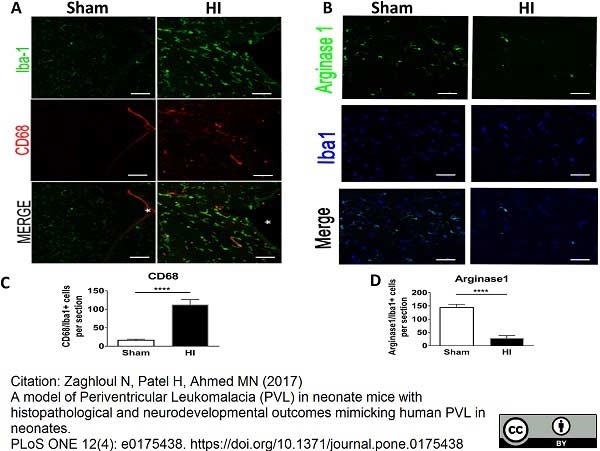
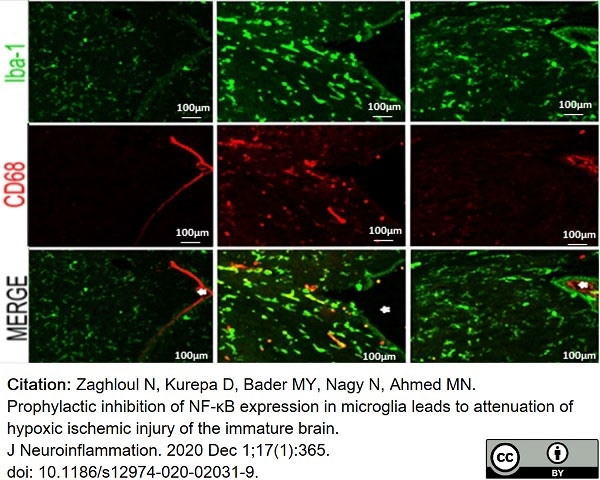
Zaghloul, N. et al. (2020) Prophylactic inhibition of NF-κB expression in microglia leads to attenuation of hypoxic ischemic injury of the immature brain.
J Neuroinflammation. 17 (1): 365. -
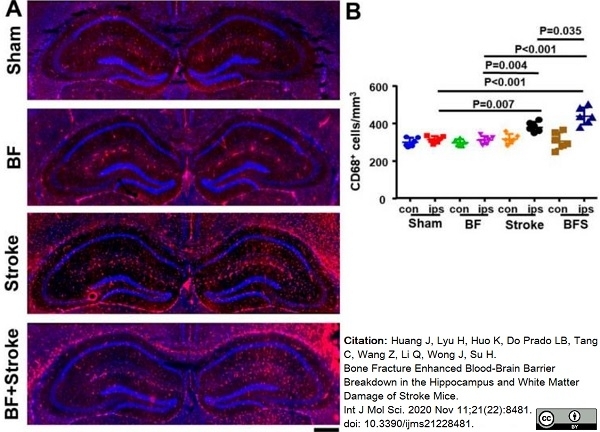
Huang, J. et al. (2020) Bone Fracture Enhanced Blood-Brain Barrier Breakdown in the Hippocampus and White Matter Damage of Stroke Mice.
Int J Mol Sci.21(22):8481. -
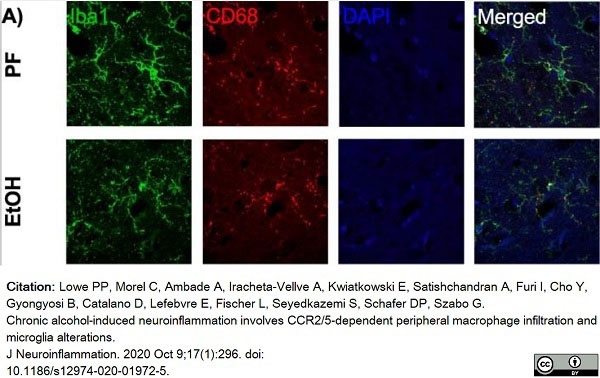
Lowe, P.P. et al. (2020) Chronic alcohol-induced neuroinflammation involves CCR2/5-dependent peripheral macrophage infiltration and microglia alterations.
J Neuroinflammation. 17 (1): 296. -
Stroobants, S. et al. (2020) Aged Tmem106b knockout mice display gait deficits in coincidence with Purkinje cell loss and only limited signs of non-motor dysfunction.
Brain Pathol. : e12903. -
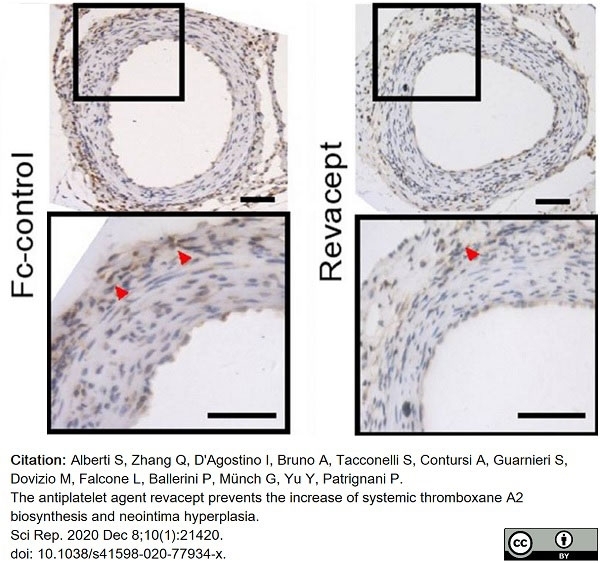
Alberti, S. et al. (2020) The antiplatelet agent revacept prevents the increase of systemic thromboxane A2 biosynthesis and neointima hyperplasia.
Sci Rep. 10 (1): 21420. -
Allen, B.D. et al. (2020) Mitigation of helium irradiation-induced brain injury by microglia depletion.
J Neuroinflammation. 17 (1): 159. -
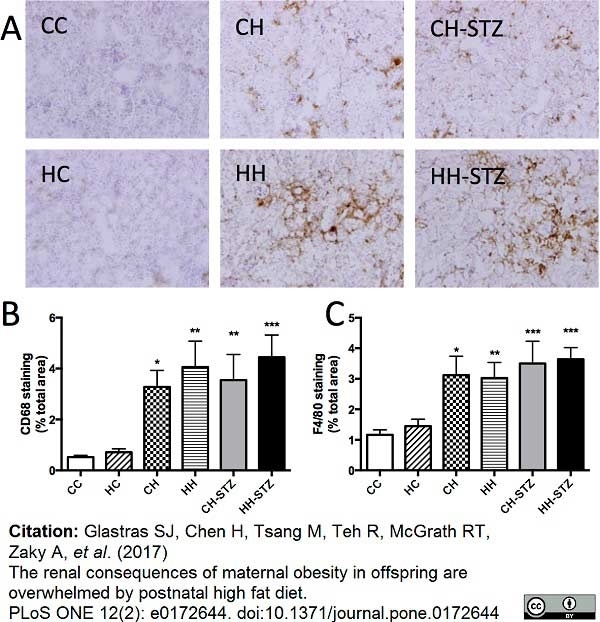
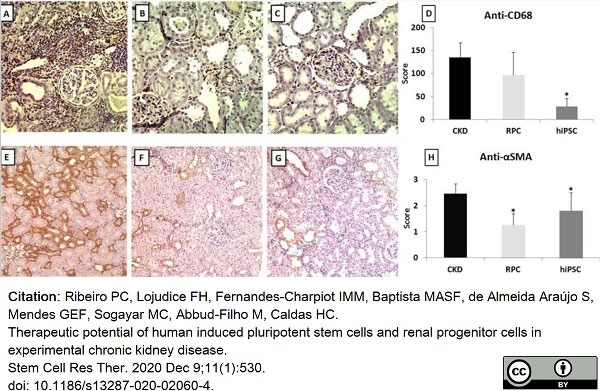
Ribeiro, P.C. et al. (2020) Therapeutic potential of human induced pluripotent stem cells and renal progenitor cells in experimental chronic kidney disease.
Stem Cell Res Ther. 11 (1): 530. -
Miró, L. et al. (2020) Dietary Supplementation with Spray-Dried Porcine Plasma Attenuates Colon Inflammation in a Genetic Mouse Model of Inflammatory Bowel Disease.
Int J Mol Sci. 21(18): 6760. -
Zhou, F. et al. (2020) β-Carotene conversion to vitamin A delays atherosclerosis progression by decreasing hepatic lipid secretion in mice.
J Lipid Res. 61 (11): 1491-1503. -
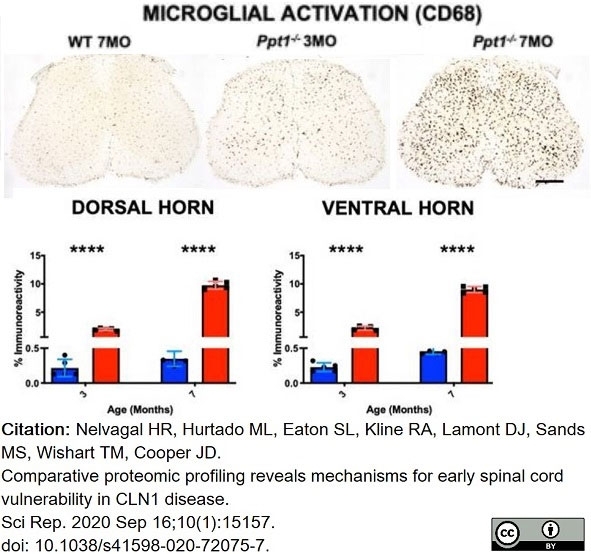
Nelvagal, H.R. et al. (2020) Comparative proteomic profiling reveals mechanisms for early spinal cord vulnerability in CLN1 disease.
Sci Rep. 10 (1): 15157. -
Grubišić, V. et al. (2020) Enteric Glia Modulate Macrophage Phenotype and Visceral Sensitivity following Inflammation.
Cell Rep. 32 (10): 108100. -
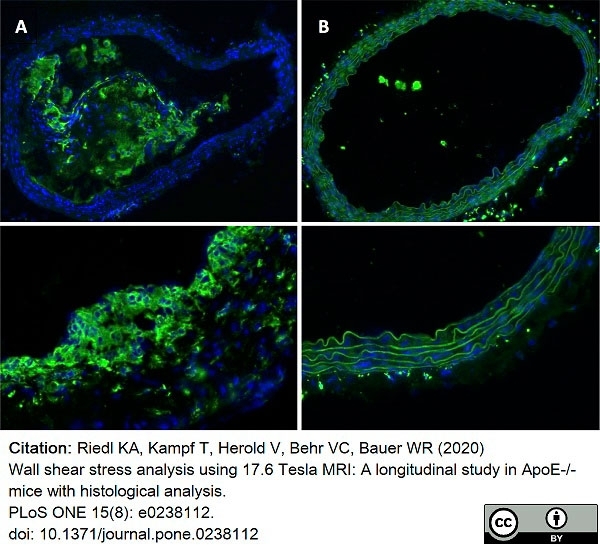
Riedl, K.A. et al. (2020) Wall shear stress analysis using 17.6 Tesla MRI: A longitudinal study in ApoE-/- mice with histological analysis.
PLoS One. 15 (8): e0238112. -
Gómez-Almería, M. et al. (2021) BiP Heterozigosity Aggravates Pathological Deterioration in Experimental Amyotrophic Lateral Sclerosis.
Int J Mol Sci. 22(22):12533. -
Freeley, S.J. et al. (2022) The lectin pathway does not contribute to glomerular injury in the nephrotoxic nephritis model.
Nephrology (Carlton). 27 (2): 208-14. -
Bourel, J. et al. (2021) Complement C3 mediates early hippocampal neurodegeneration and memory impairment in experimental multiple sclerosis
Neurobiology of Disease. 160: 105533. -
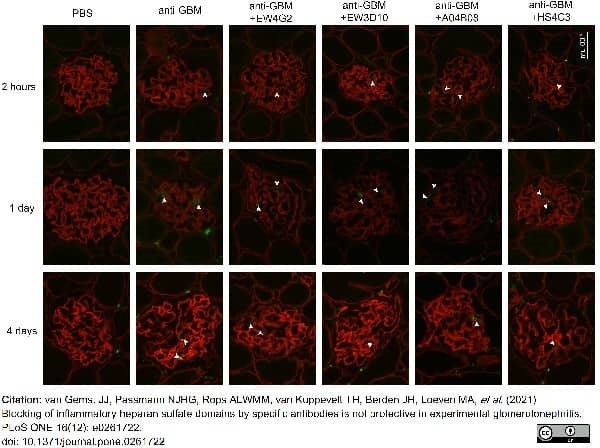
van Gemst, J.J. et al. (2021) Blocking of inflammatory heparan sulfate domains by specific antibodies is not protective in experimental glomerulonephritis.
PLoS One. 16 (12): e0261722. -
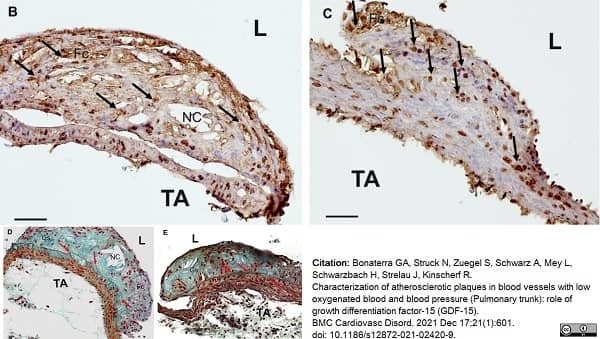
Bonaterra, G.A. et al. (2021) Characterization of atherosclerotic plaques in blood vessels with low oxygenated blood and blood pressure (Pulmonary trunk): role of growth differentiation factor-15 (GDF-15)
BMC Cardiovascular Disorders. 21: 601. -
Venturino, A. & Siegert, S. (2021) Minimally invasive protocols and quantification for microglia-mediated perineuronal net disassembly in mouse brain
STAR Protocols. 2 (4): 101012. -
Perez-Canamas, A. et al. (2021) Fronto-temporal dementia risk gene TMEM106B has opposing effects in different lysosomal storage disorders.
Brain Commun. 3 (1): fcaa200. -
Stavropoulos, F. et al. (2021) Aberrant Mitochondrial Dynamics and Exacerbated Response to Neuroinflammation in a Novel Mouse Model of CMT2A.
Int J Mol Sci. 22 (21): 11569. -
He, D. et al. (2021) Disruption of the IL-33-ST2-AKT signaling axis impairs neurodevelopment by inhibiting microglial metabolic adaptation and phagocytic function.
Immunity. S1074-7613(21)00534-3. -
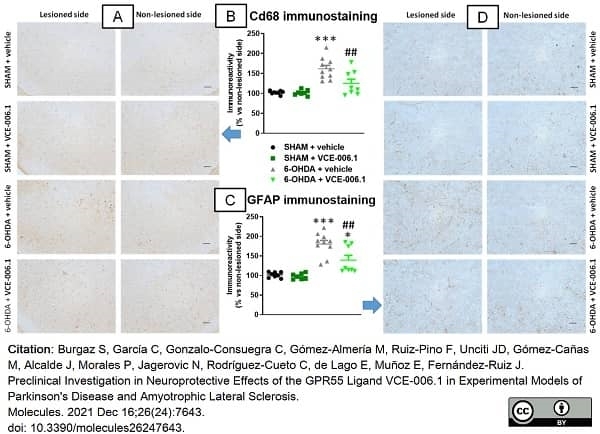
Burgaz, S. et al. (2021) Preclinical Investigation in Neuroprotective Effects of the GPR55 Ligand VCE-006.1 in Experimental Models of Parkinson's Disease and Amyotrophic Lateral Sclerosis.
Molecules. 26 (24):7643. -
Xiong, L. et al. (2021) Inflammation-dependent oxidative stress metabolites as a hallmark of amyotrophic lateral sclerosis.
Free Radic Biol Med. 178: 125-33. -
Zhao, L.X. et al. (2021) TLR8 in the Trigeminal Ganglion Contributes to the Maintenance of Trigeminal Neuropathic Pain in Mice.
Neurosci Bull. 37 (4): 550-62. -
Rothe, R. et al. (2021) A modular, injectable, non-covalently assembled hydrogel system features widescale tunable degradability for controlled release and tissue integration.
Biomaterials. 269: 120637. -
Gratuze, M. et al. (2021) Activated microglia mitigate Aβ-associated tau seeding and spreading.
J Exp Med. 218(8):e20210542. -
Merlin, J. et al. (2021) Non-canonical glutamine transamination sustains efferocytosis by coupling redox buffering to oxidative phosphorylation.
Nat Metab. 3 (10): 1313-26. -
Tang, X. et al. (2021) Aloe-emodin derivative produces anti-atherosclerosis effect by reinforcing AMBRA1-mediated endothelial autophagy.
Eur J Pharmacol.2021 Nov 17 : 174641. -
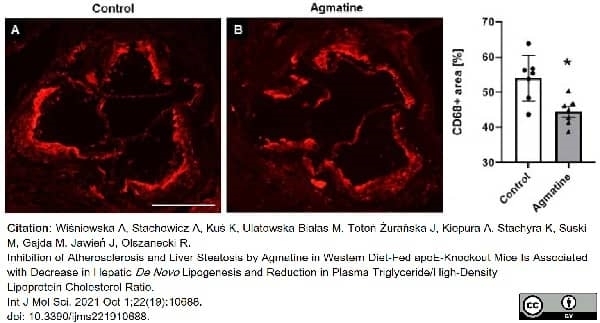
Wiśniewska, A. et al. (2021) Inhibition of Atherosclerosis and Liver Steatosis by Agmatine in Western Diet-Fed apoE-Knockout Mice Is Associated with Decrease in Hepatic De Novo. Lipogenesis and Reduction in Plasma Triglyceride/High-Density Lipoprotein Cholesterol Ratio.
Int J Mol Sci.22 (19): 10688. -
Shi, Q. et al. (2021) Ultrasound-mediated blood-brain barrier disruption improves anti-pyroglutamate3 Aβ antibody efficacy and enhances phagocyte infiltration into brain in aged Alzheimer’s disease-like mice
bioRxiv preprint: Jan 17 [Epub ahead of print]. -
Cheah, F.C. et al. (2021) Studying the Effects of Granulocyte-Macrophage Colony-Stimulating Factor on Fetal Lung Macrophages During the Perinatal Period Using the Mouse Model.
Front Pediatr. 9: 614209. -
Dorighello, G.G. et al. (2021) Mild Mitochondrial Uncoupling Decreases Experimental Atherosclerosis, A Proof of Concept.
J Atheroscler Thromb. 29 (6): 825-38. -
Alam, M.M. et al. (2021) Deficiency of microglial autophagy increases the density of oligodendrocytes and susceptibility to severe forms of seizures.
eNeuro.;8 (1): ENEURO.0183-20.2021. -
Vandestienne, M. et al. (2021) TREM-1 orchestrates angiotensin II-induced monocyte trafficking and promotes experimental abdominal aortic aneurysm.
J Clin Invest. 131 (2): e142468. -
Apodaca, L.A. et al. (2021) Human neural stem cell-derived extracellular vesicles mitigate hallmarks of Alzheimer's disease.
Alzheimers Res Ther. 13 (1): 57. -
Gajeton, J. et al. (2021) Hyperglycemia-Induced miR-467 Drives Tumor Inflammation and Growth in Breast Cancer.
Cancers (Basel). 13(6):1346. -
Leipner, J. et al. (2021) Myeloid cell-specific Irf5 deficiency stabilizes atherosclerotic plaques in Apoe-/- mice.
Mol Metab. 53:101250. -
Shariq, M. et al. (2021) Adult neural stem cells have latent inflammatory potential that is kept suppressed by Tcf4 to facilitate adult neurogenesis.
Sci Adv. 7 (21): eabf5606. -
Martínez-Beamonte, R. et al. (2021) Dietary Avian Proteins Are Comparable to Soybean Proteins on the Atherosclerosis Development and Fatty Liver Disease in Apoe-Deficient Mice
Nutrients. 13 (6): 1838. -
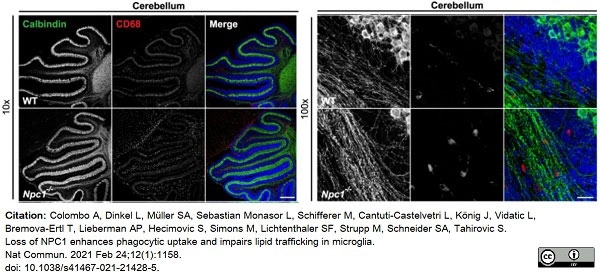
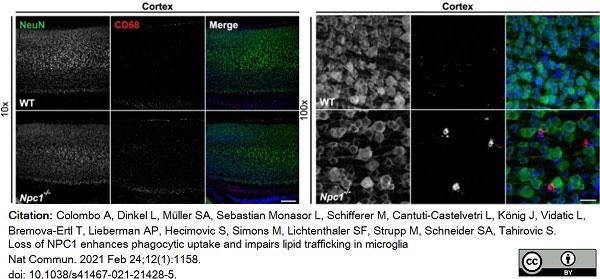
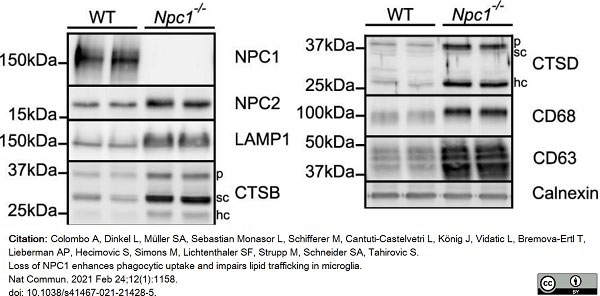
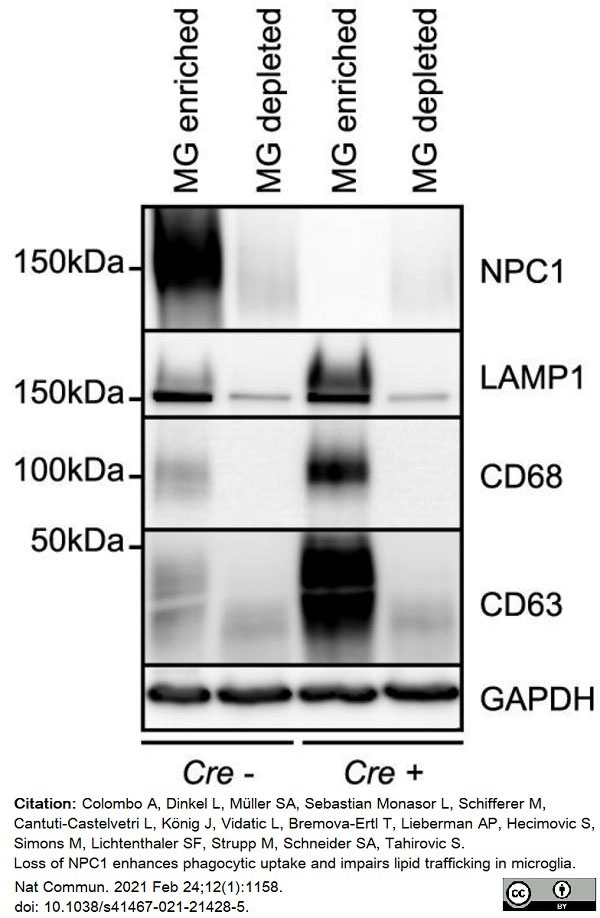
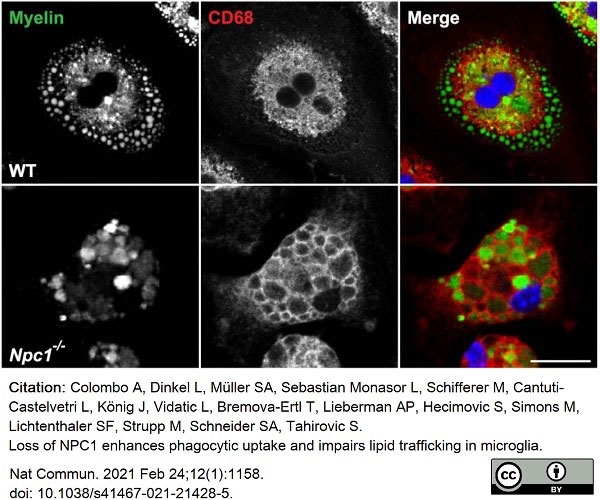
Colombo, A. et al. (2021) Loss of NPC1 enhances phagocytic uptake and impairs lipid trafficking in microglia.
Nat Commun. 12 (1): 1158. -
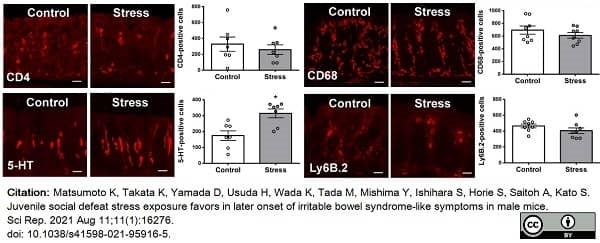
Matsumoto, K. et al. (2021) Juvenile social defeat stress exposure favors in later onset of irritable bowel syndrome-like symptoms in male mice.
Sci Rep. 11 (1): 16276. -
Nakata, Y. et al. (2021) Role of podoplanin and Kupffer cells in liver injury after ischemia-reperfusion in mice.
Surg Today. 52(2):344-53. -
Colombo, A.V. et al. (2021) Microbiota-derived short chain fatty acids modulate microglia and promote Aβ plaque deposition.
Elife. 10:e59826. -
Flores, I. et al. (2021) Myeloid cell-mediated targeting of LIF to dystrophic muscle causes transient increases in muscle fiber lesions by disrupting the recruitment and dispersion of macrophages in muscle.
Hum Mol Genet. 31(2):189-206. -
Park, G.T. et al. (2021) Echinochrome A Treatment Alleviates Fibrosis and Inflammation in Bleomycin-Induced Scleroderma.
Mar Drugs. 19 (5): 237. -
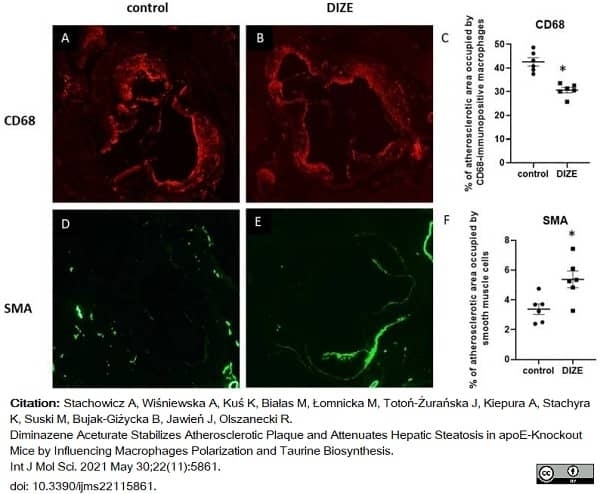
Stachowicz, A. et al. (2021) Diminazene Aceturate Stabilizes Atherosclerotic Plaque and Attenuates Hepatic Steatosis in apoE-Knockout Mice by Influencing Macrophages Polarization and Taurine Biosynthesis.
Int J Mol Sci. 22 (11): 5861 -
Shahraz, A. et al. (2021) Phagocytosis-related NADPH oxidase 2 subunit gp91phox contributes to neurodegeneration after repeated systemic challenge with lipopolysaccharides.
Glia. 69 (1): 137-50. -
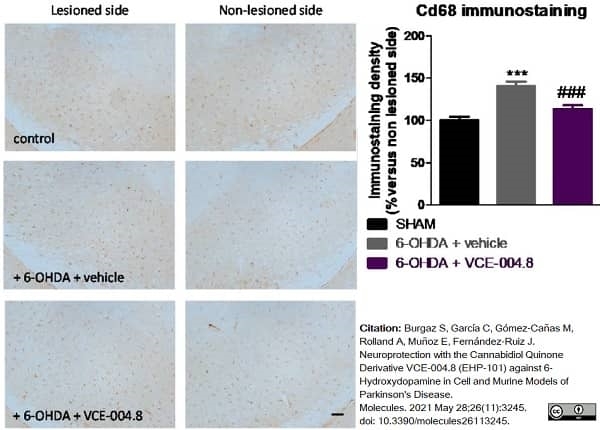
Burgaz, S. et al. (2021) Neuroprotection with the Cannabidiol Quinone Derivative VCE-004.8 (EHP-101) against 6-Hydroxydopamine in Cell and Murine Models of Parkinson's Disease.
Molecules. 26 (11): 3245. -
Martínez-Beamonte, R. et al. (2021) Dietary Avian Proteins Are Comparable to Soybean Proteins on the Atherosclerosis Development and Fatty Liver Disease in Apoe-Deficient Mice.
Nutrients. 13 (6):1838. -
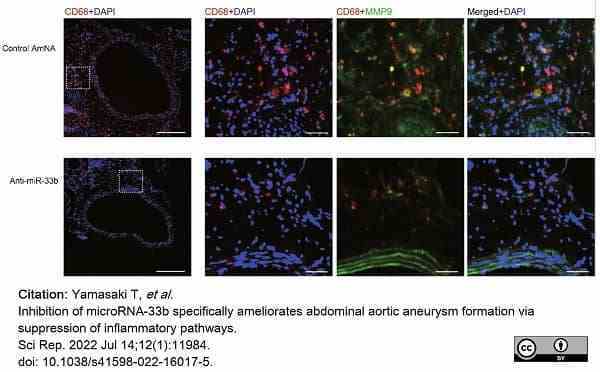
Yamasaki, T. et al. (2022) Inhibition of microRNA-33b specifically ameliorates abdominal aortic aneurysm formation via suppression of inflammatory pathways.
Sci Rep. 12 (1): 11984. -
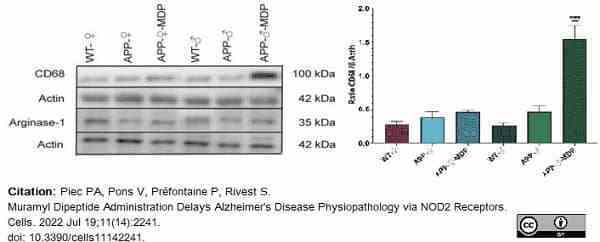
Piec, P.A. et al. (2022) Muramyl Dipeptide Administration Delays Alzheimer's Disease Physiopathology via NOD2 Receptors.
Cells. 11 (14): 2241. -
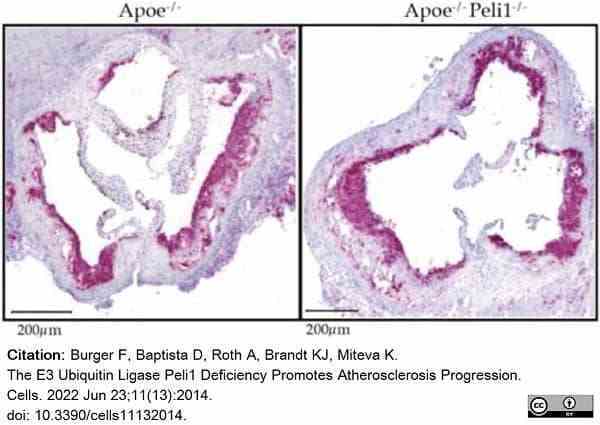
Burger, F. et al. (2022) The E3 Ubiquitin Ligase Peli1 Deficiency Promotes Atherosclerosis Progression.
Cells. 11 (13): 2014. -
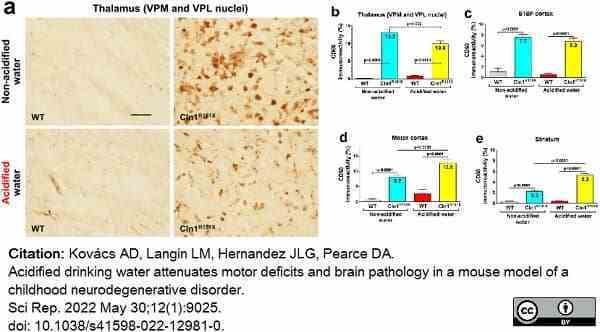
Kovács, A.D. et al. (2022) Acidified drinking water attenuates motor deficits and brain pathology in a mouse model of a childhood neurodegenerative disorder.
Sci Rep. 12 (1): 9025. -
Miller-Rhodes, P. et al. (2022) URMC-099 prophylaxis prevents hippocampal vascular vulnerability and synaptic damage in an orthopedic model of delirium superimposed on dementia.
FASEB J. 36 (6): e22343. -
Bardin, M. et al. (2022) The resolvin D2 - GPR18 axis is expressed in human coronary atherosclerosis and transduces atheroprotection in apolipoprotein E deficient mice.
Biochem Pharmacol. : 115075. -
Wang, Y.Y. et al. (2022) Loss of microglial EED impairs synapse density, learning, and memory.
27(7):2999-3009. -
Poggi, G. et al. (2022) Effects of chronic social stress on oligodendrocyte proliferation-maturation and myelin status in prefrontal cortex and amygdala in adult mice
Neurobiol Stress. 18: 100451. -
Enrich-Bengoa, J. et al. (2022) TRPV2: A Key Player in Myelination Disorders of the Central Nervous System.
Int J Mol Sci. 23 (7): 3617. -
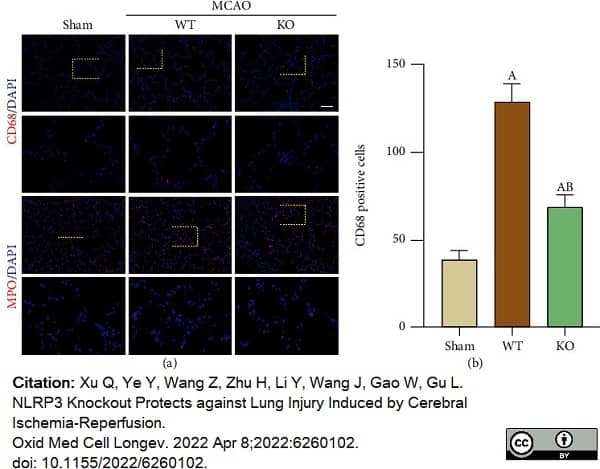
Xu, Q. et al. (2022) NLRP3 Knockout Protects against Lung Injury Induced by Cerebral Ischemia-Reperfusion.
Oxid Med Cell Longev. 2022: 6260102. -
Liu, J. et al. (2022) Pregnane X Receptor Mediates Atherosclerosis Induced by Dicyclohexyl Phthalate in LDL Receptor-Deficient Mice
Cells. 11 (7): 1125 -
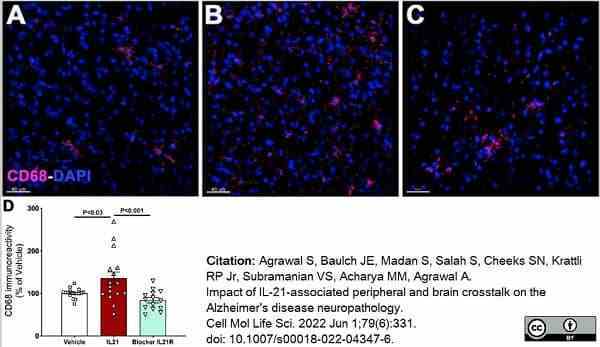
Agrawal, S. et al. (2022) Impact of IL-21-associated peripheral and brain crosstalk on the Alzheimer's disease neuropathology.
Cell Mol Life Sci. 79 (6): 331. -
Lam, S. et al. (2022) Pathological changes induced by Alzheimer's brain inoculation in amyloid-beta plaque-bearing mice.
Acta Neuropathol Commun. 10 (1): 112. -
Qiao, S. et al. (2022) Gut Parabacteroides merdae protects against cardiovascular damage by enhancing branched-chain amino acid catabolism.
Nat Metab. 4 (10): 1271-86. -
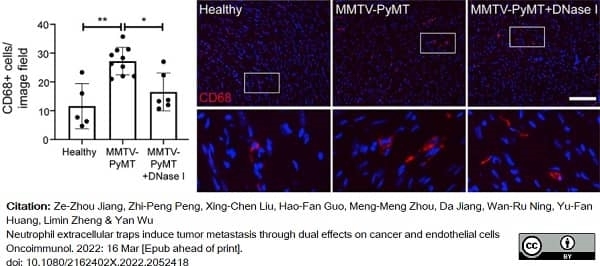
Cedervall, J. et al. (2022) Neutrophil extracellular traps promote cancer-associated inflammation and myocardial stress.
Oncoimmunology. 11 (1): 2049487. -
Albertini, G. et al. (2022) Serotonin sensing by microglia conditions the proper development of neuronal circuits and of social and adaptive skills
bioRxiv Mar 10 [Epub ahead of print]. -
Burger, F. et al. (2022) Single-Cell RNA-Seq Reveals a Crosstalk between Hyaluronan Receptor LYVE-1-Expressing Macrophages and Vascular Smooth Muscle Cells.
Cells. 11 (3): 411. -
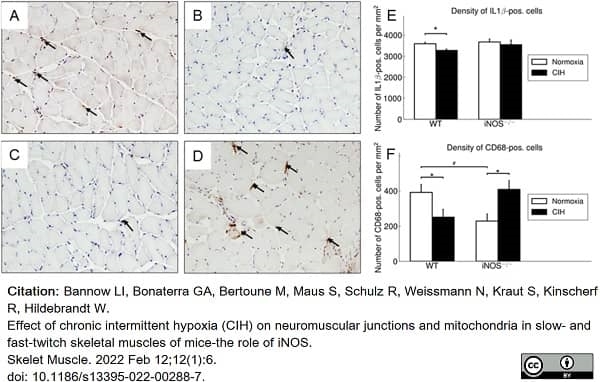
Bannow, L.I. et al. (2022) Effect of chronic intermittent hypoxia (CIH) on neuromuscular junctions and mitochondria in slow- and fast-twitch skeletal muscles of mice-the role of iNOS.
Skelet Muscle. 12 (1): 6. -
Bey, L. et al. (2022) TCDD aggravates the formation of the atherosclerotic plaque in ApoE KO mice with a sexual dimorphic pattern.
Biochimie. 195: 54-58. -
Chute, M. et al. (2022) ADAM15 is required for optimal collagen cross-linking and scar formation following myocardial infarction.
Matrix Biol. 105: 127-43. -
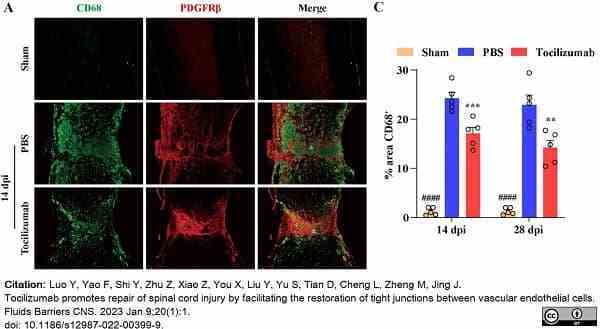
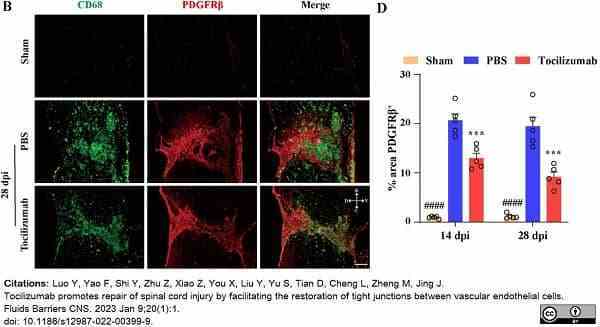
Luo, Y. et al. (2022) M1 macrophages impair tight junctions between endothelial cells after spinal cord injury.
Brain Res Bull. 180: 59-72. -
Jia, W. et al. (2022) MircoRNA-126-5p inhibits apoptosis of endothelial cell in vascular arterial walls via NF-κB/PI3K/AKT/mTOR signaling pathway in atherosclerosis.
J Mol Histol. 53 (1): 51-62. -
Lee, H.J. et al. (2022) Effects of electroacupuncture on the functionality of NG2-expressing cells in perilesional brain tissue of mice following ischemic stroke.
Neural Regen Res. 17 (7): 1556-65. -
Palandri, A. et al. (2022) Ablation of arginyl-tRNA-protein transferase in oligodendrocytes impairs central nervous system myelination.
Glia. 70 (2): 303-20. -
Cheng, J. et al. (2022) Unilateral Sciatic Nerve Crush Induces White Blood Cell Infiltration of the Contralateral Nerve.
J Healthc Eng. 2022: 1101383. -
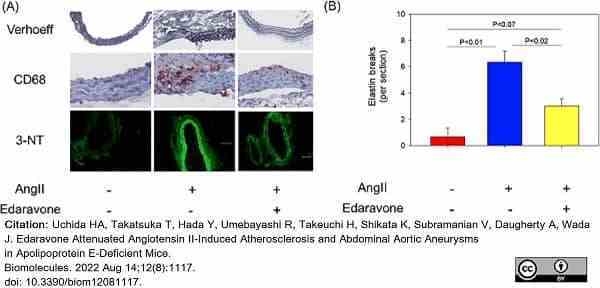
Uchida, H.A. et al. (2022) Edaravone Attenuated Angiotensin II-Induced Atherosclerosis and Abdominal Aortic Aneurysms in Apolipoprotein E-Deficient Mice.
Biomolecules. 12(8):1117. -
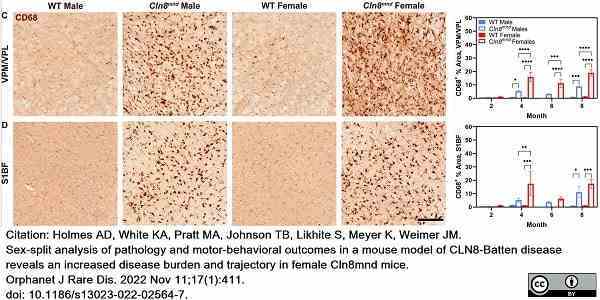
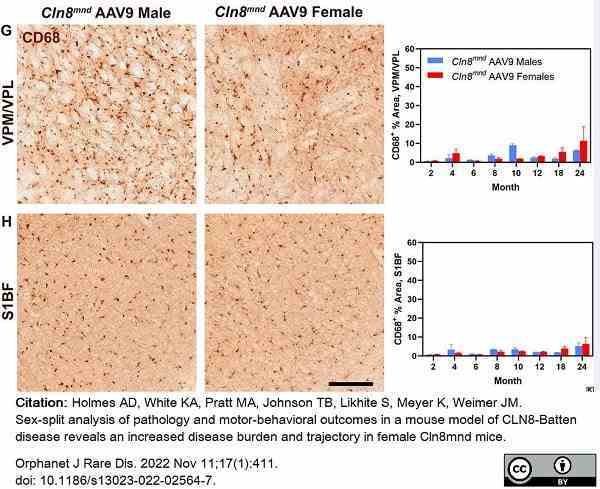
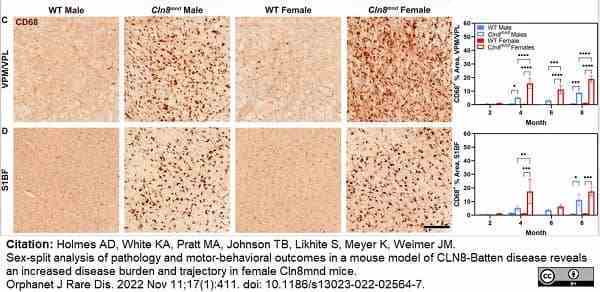
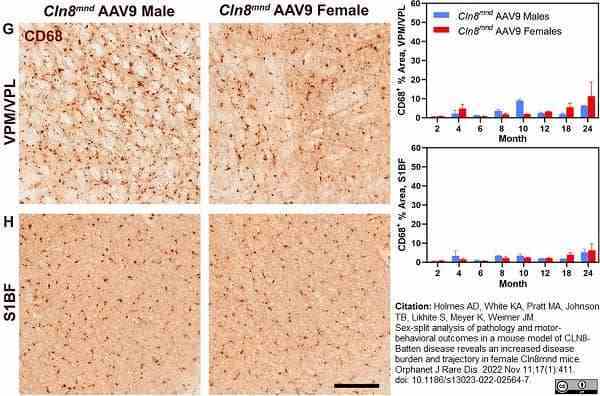
Holmes, A.D. et al. (2022) Sex-split analysis of pathology and motor-behavioral outcomes in a mouse model of CLN8-Batten disease reveals an increased disease burden and trajectory in female Cln8(mnd) mice.
Orphanet J Rare Dis. 17 (1): 411. -
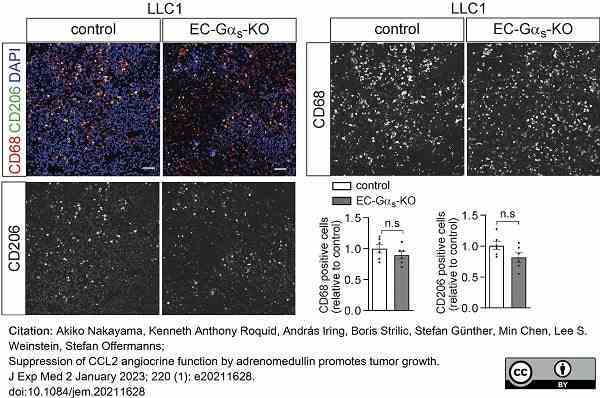
Nakayama, A. et al. (2023) Suppression of CCL2 angiocrine function by adrenomedullin promotes tumor growth
J Exp Med. 220 (1): e20211628. -
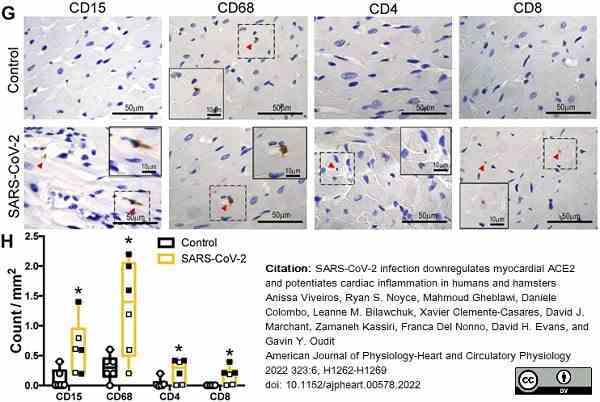
Viveiros, A. et al. (2022) SARS-CoV-2 infection downregulates myocardial ACE2 and potentiates cardiac inflammation in humans and hamsters.
Am J Physiol Heart Circ Physiol. 323 (6): H1262-H1269. -
Luo, Y. et al. (2023) Tocilizumab promotes repair of spinal cord injury by facilitating the restoration of tight junctions between vascular endothelial cells.
Fluids Barriers CNS. 20 (1): 1. -
Endo, M. et al. (2022) Hangekobokuto, a traditional Japanese herbal medicine, ameliorates postoperative ileus through its anti-inflammatory action.
J Smooth Muscle Res. 58: 78-88. -
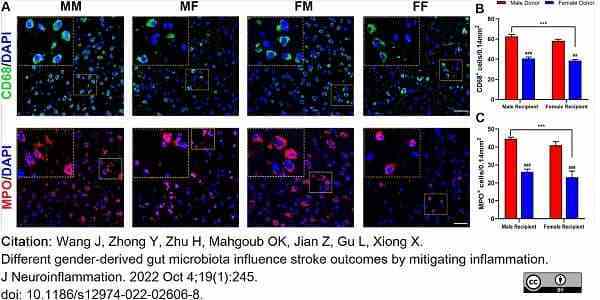
Wang, J. et al. (2022) Different gender-derived gut microbiota influence stroke outcomes by mitigating inflammation.
J Neuroinflammation. 19 (1): 245. -
van Os E.A. et al. (2022) Modelling fatty liver disease with mouse liver-derived multicellular spheroids.
Biomaterials. 290: 121817. -
Baytas, O. et al. (2022) Loss of mitochondrial enzyme GPT2 causes early neurodegeneration in locus coeruleus.
Neurobiol Dis. 173: 105831. -
Colombo, G. et al. (2022) A tool for mapping microglial morphology, morphOMICs, reveals brain-region and sex-dependent phenotypes.
Nat Neurosci. 25 (10): 1379-93. -
Florez-Barros, F. et al. (2023) Myeloid expression of the anti-apoptotic protein Mcl1 is required in anti-myeloperoxidase vasculitis but myeloperoxidase inhibition is not protective.
Kidney Int. 103 (1): 134-3. -
Liu, Y. et al. (2022) LRRK2 deficiency protects the heart against myocardial infarction injury in mice via the P53/HMGB1 pathway.
Free Radic Biol Med. 191: 119-27. -
Ma, C. et al. (2020) Arginase 1 Insufficiency Precipitates Amyloid-β Deposition and Hastens Behavioral Impairment in a Mouse Model of Amyloidosis.
Front Immunol. 11: 582998. -
Wang, J. et al. (2022) YAP1 protects against septic liver injury via ferroptosis resistance.
Cell Biosci. 12 (1): 163. -
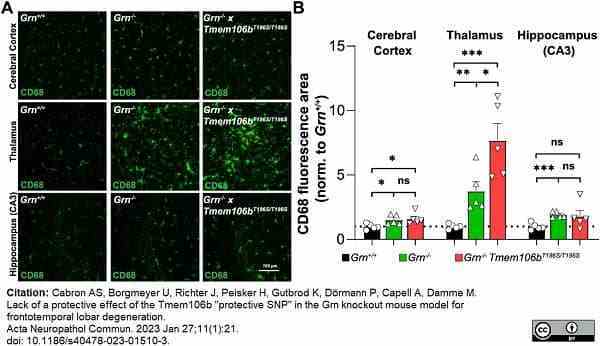
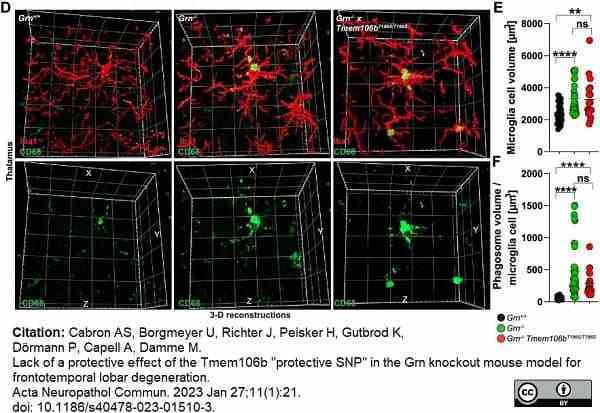
Cabron, A.S. et al. (2023) Lack of a protective effect of the Tmem106b "protective SNP" in the Grn knockout mouse model for frontotemporal lobar degeneration.
Acta Neuropathol Commun. 11 (1): 21. -
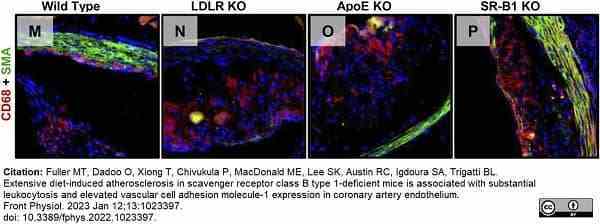
Fuller, M.T. et al. (2022) Extensive diet-induced atherosclerosis in scavenger receptor class B type 1-deficient mice is associated with substantial leukocytosis and elevated vascular cell adhesion molecule-1 expression in coronary artery endothelium.
Front Physiol. 13: 1023397. -
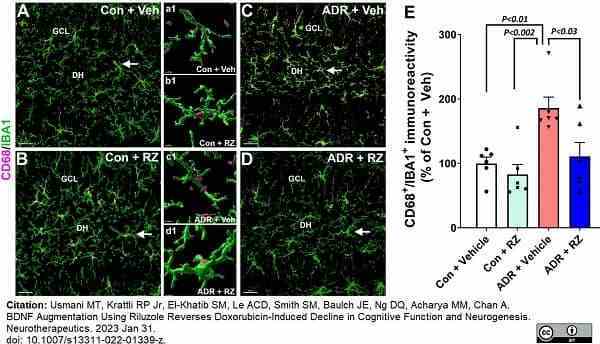
Usmani, M.T. et al. (2023) BDNF Augmentation Using Riluzole Reverses Doxorubicin-Induced Decline in Cognitive Function and Neurogenesis.
Neurotherapeutics. Jan 31 [Epub ahead of print]. -
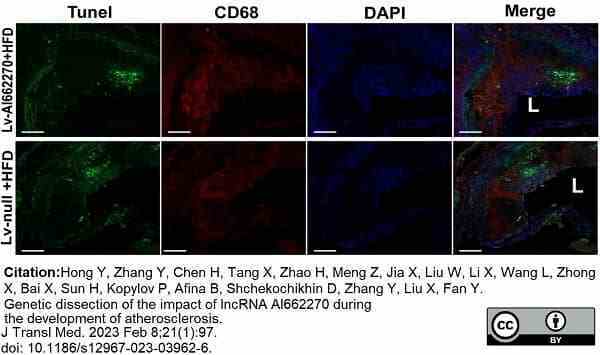
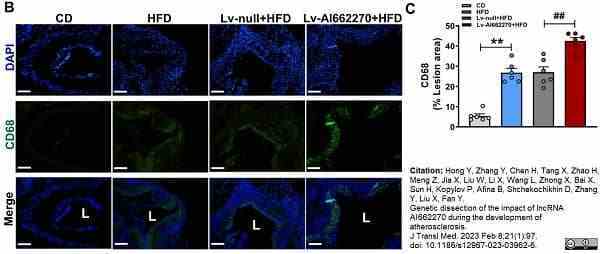
Hong, Y. et al. (2023) Genetic dissection of the impact of lncRNA AI662270 during the development of atherosclerosis.
J Transl Med. 21 (1): 97. -
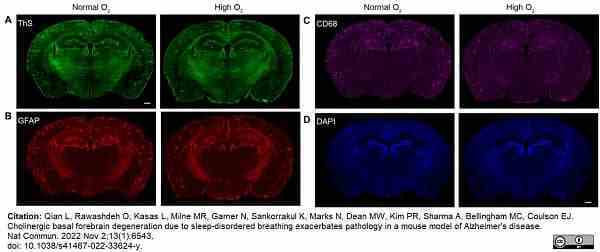
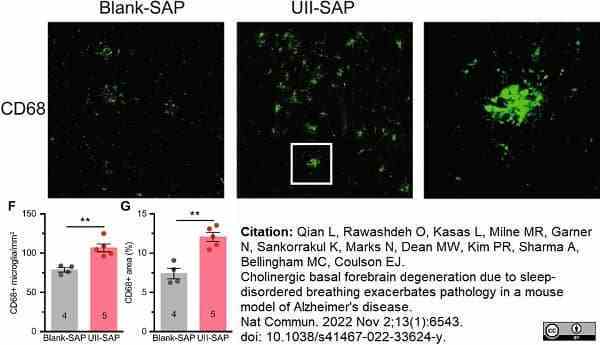
Qian, L. et al. (2022) Cholinergic basal forebrain degeneration due to sleep-disordered breathing exacerbates pathology in a mouse model of Alzheimer's disease.
Nat Commun. 13 (1): 6543. -
Zheng, W. et al. (2023) Mycobacterium tuberculosis resides in lysosome-poor monocyte-derived lung cells during persistent infection.
bioRxiv. Jan 20 [Epub ahead of print]. -
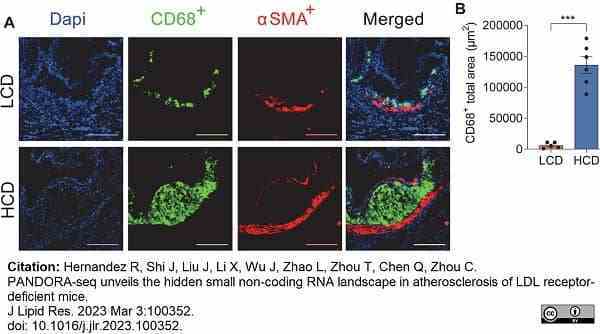
Hernandez, R. et al. (2023) PANDORA-seq unveils the hidden small non-coding RNA landscape in atherosclerosis of LDL receptor-deficient mice.
J Lipid Res. : 100352. -
Spella, M. et al. (2023) Non-Oncogene Addiction of KRAS-Mutant Cancers to IL-1β via Versican and Mononuclear IKKβ.
Cancers. 15 (6): 1866. -
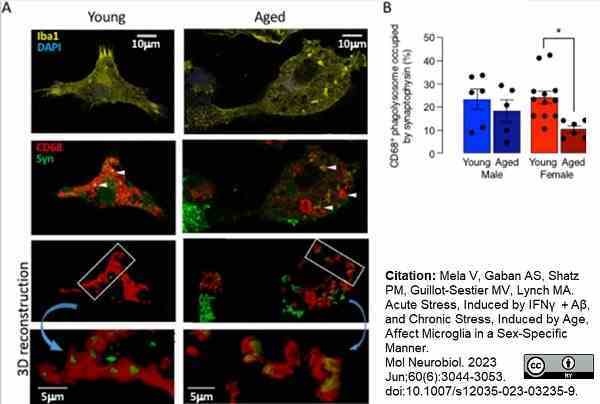
Mela, V. et al. (2023) Acute Stress, Induced by IFNγ + Aβ, and Chronic Stress, Induced by Age, Affect Microglia in a Sex-Specific Manner.
Mol Neurobiol. 60 (6): 3044-53. -
Härdtner, C. et al. (2023) A comparative gene expression matrix in Apoe-deficient mice identifies unique and atherosclerotic disease stage-specific gene regulation patterns in monocytes and macrophages.
Atherosclerosis. 371: 1-13. -
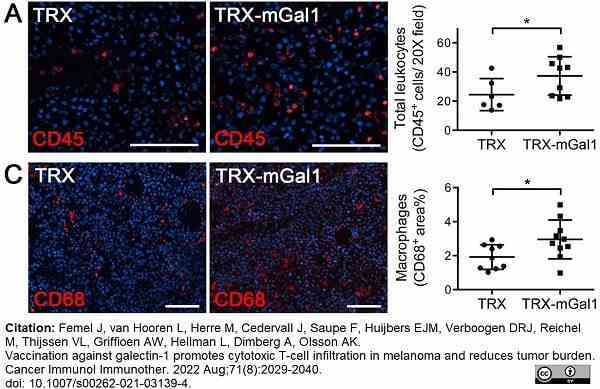
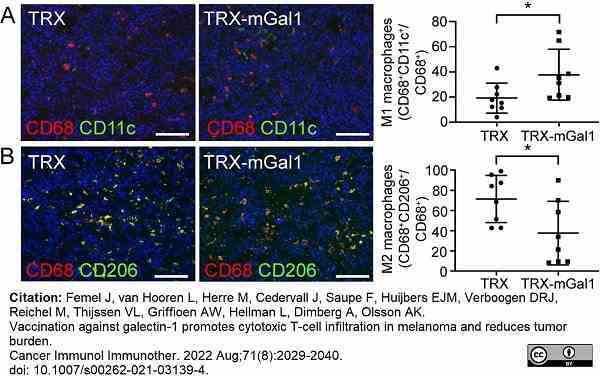
Femel, J. et al. (2022) Vaccination against galectin-1 promotes cytotoxic T-cell infiltration in melanoma and reduces tumor burden.
Cancer Immunol Immunother. 71 (8): 2029-40. -
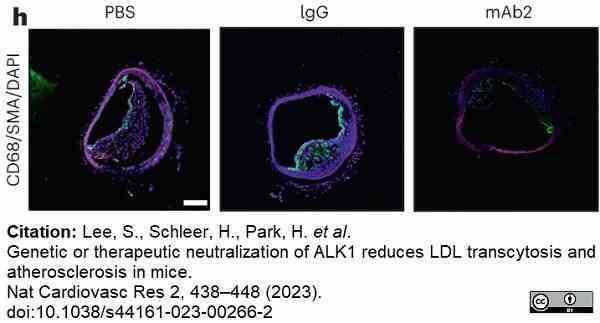
Lee, S. et al. (2023) Genetic or therapeutic neutralization of ALK1 reduces LDL transcytosis and atherosclerosis in mice.
Nat Cardiovasc Res. 2 (5): 438-48. -
Peppercorn, K. et al. (2023) Secreted Amyloid Precursor Protein Alpha (sAPPα) Regulates the Cellular Proteome and Secretome of Mouse Primary Astrocytes.
Int J Mol Sci. 24 (8): 7165. -
Scaricamazza, S. et al. (2022) Repurposing of Trimetazidine for amyotrophic lateral sclerosis: A study in SOD1(G93A) mice.
Br J Pharmacol. 179 (8): 1732-52. -
Arndt, L. et al. (2023) Trib1 Deficiency Promotes Hyperlipidemia, Inflammation, and Atherosclerosis in LDL Receptor Knockout Mice.
Arterioscler Thromb Vasc Biol. 43 (6): 979-94. -
Mitsui, S. et al. (2023) TUNEL-positive structures in activated microglia and SQSTM1/p62-positive structures in activated astrocytes in the neurodegenerative brain of a CLN10 mouse model.
Glia. 71 (12): 2753-69. -
Rodríguez-Carreiro, S. (2023) The Cannabigerol Derivative VCE-003.2 Exerts Therapeutic Effects in 6-Hydroxydopamine-Lesioned Mice: Comparison with The Classic Dopaminergic Replacement Therapy.
Brain Sci. 13 (9):1272. -
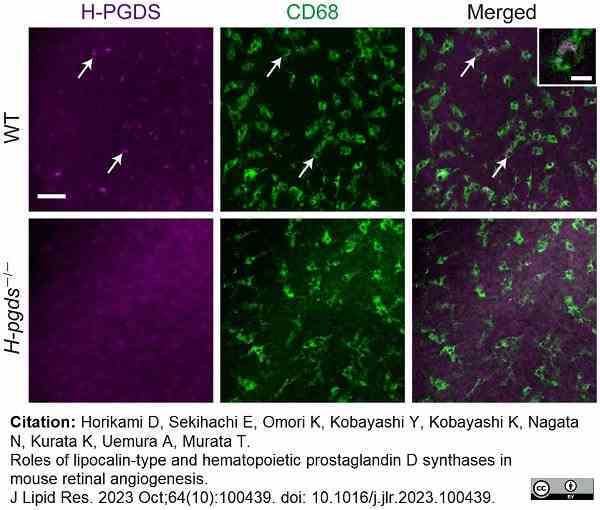
Horikami, D. (2023) Roles of lipocalin-type and hematopoietic prostaglandin D synthases in mouse retinal angiogenesis.
J Lipid Res. 64 (10):100439. -
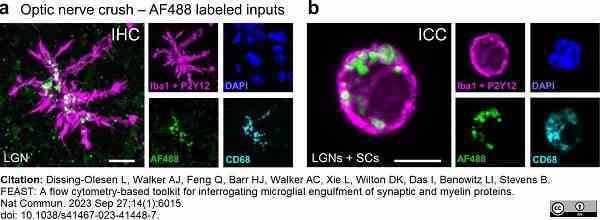
Dissing-Olesen, L. et al. (2023) FEAST: A flow cytometry-based toolkit for interrogating microglial engulfment of synaptic and myelin proteins.
Nat Commun. 14 (1): 6015. -
Wilton, D.K. et al. (2023) Microglia and complement mediate early corticostriatal synapse loss and cognitive dysfunction in Huntington's disease.
Nat Med. Oct 09 [Epub ahead of print]. -
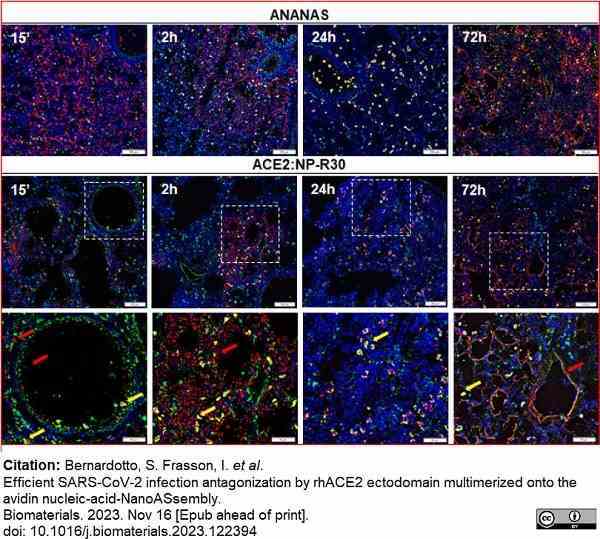
Bernardotto, S. et al. (2023) Efficient SARS-CoV-2 infection antagonization by rhACE2 ectodomain multimerized onto the avidin nucleic-acid-NanoASsembly
Biomaterials. : 122394. -
Peviani, M. et al. (2023) An innovative hematopoietic stem cell gene therapy approach benefits CLN1 disease in the mouse model.
EMBO Mol Med. 15 (4): e15968. -
Wiśniewska, A. et al. (2023) The antiatherosclerotic action of 1G244 - An inhibitor of dipeptidyl peptidases 8/9 - is mediated by the induction of macrophage death.
Eur J Pharmacol. 944: 175566. -
Zhang, X. et al. (2023) Loss of Macrophage mTORC2 Drives Atherosclerosis via FoxO1 and IL-1β Signaling.
Circ Res. 133 (3): 200-19. -
Schlundt, C. et al. (2023) Complex Spatio-Temporal Interplay of Distinct Immune and Bone Cell Subsets during Bone Fracture Healing
Cells. 13 (1): 40. -
Wang, C. et al. (2021) Selective removal of astrocytic APOE4 strongly protects against tau-mediated neurodegeneration and decreases synaptic phagocytosis by microglia.
Neuron. 109 (10): 1657-74.e7. -
Stobart, J.L. et al. (2018) Long-term In Vivo Calcium Imaging of Astrocytes Reveals Distinct Cellular Compartment Responses to Sensory Stimulation.
Cereb Cortex. 28 (1): 184-98. -
Stein, S. et al. (2021) Deletion of fibroblast activation protein provides atheroprotection.
Cardiovasc Res. 117 (4): 1060-9. -
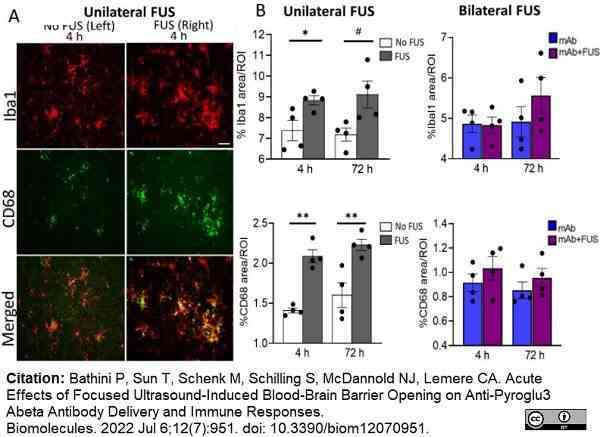
Bathini, P. et al. (2022) Acute Effects of Focused Ultrasound-Induced Blood-Brain Barrier Opening on Anti-Pyroglu3 Abeta Antibody Delivery and Immune Responses.
Biomolecules. 12 (7): 951. -
Drost, N. et al. (2020) The Amyloid-beta rich CNS environment alters myeloid cell functionality independent of their origin.
Sci Rep. 10 (1): 7152. -
El Gaamouch, F. et al. (2020) VGF-derived peptide TLQP-21 modulates microglial function through C3aR1 signaling pathways and reduces neuropathology in 5xFAD mice.
Mol Neurodegener. 15 (1): 4. -
Hoeijmakers, L. et al. (2018) The age-related slow increase in amyloid pathology in APP.V717I mice activates microglia, but does not alter hippocampal neurogenesis.
Neurobiol Aging. 61: 112-23. -
Jain, N. et al. (2023) Chronic TREM2 activation exacerbates Aβ-associated tau seeding and spreading.
J Exp Med. 220 (1): e20220654. -
Owlett, L. et al. (2020) Space radiation does not alter amyloid or tau pathology in the 3xTg mouse model of Alzheimer's disease.
Life Sci Space Res (Amst). 27: 89-98. -
Qian, L. et al. (2019) Removal of p75 Neurotrophin Receptor Expression from Cholinergic Basal Forebrain Neurons Reduces Amyloid-β Plaque Deposition and Cognitive Impairment in Aged APP/PS1 Mice.
Mol Neurobiol. 56 (7): 4639-52. -
Unger, M.S. et al. (2018) Doublecortin expression in CD8+ T-cells and microglia at sites of amyloid-β plaques: A potential role in shaping plaque pathology?
Alzheimers Dement. 14 (8): 1022-37. -
Huo, K. et al. (2021) Reduction of neuroinflammation alleviated mouse post bone fracture and stroke memory dysfunction.
J Cereb Blood Flow Metab. 41 (9): 2162-73. -
Hashemi, E. et al. (2023) Visualizing Sphingosine-1-Phosphate Receptor 1(S1P(1)) Signaling During Central Nervous System De- and Remyelination.
Cell Mol Neurobiol. 43 (3): 1219-36. -
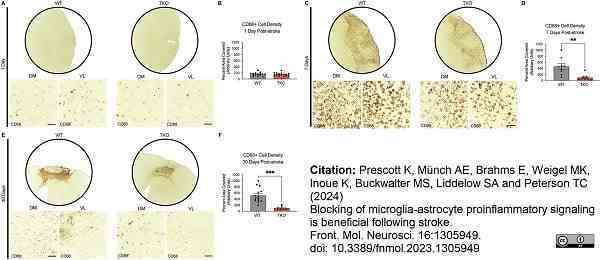
Prescott, K. et al. (2024) Blocking of microglia-astrocyte proinflammatory signaling is beneficial following stroke.
Frontiers in Molecular Neuroscience. 04 Jan [Epub ahead of print]. -
Han, X. et al. (2019) Microglial Depletion with Clodronate Liposomes Increases Proinflammatory Cytokine Levels, Induces Astrocyte Activation, and Damages Blood Vessel Integrity.
Mol Neurobiol. 56 (9): 6184-96. -
van Olst,.L. et al. (2020) Microglial activation arises after aggregation of phosphorylated-tau in a neuron-specific P301S tauopathy mouse model.
Neurobiol Aging. 89: 89-98. -
Fotio, Y. et al. (2024) NAAA-regulated lipid signaling in monocytes controls the induction of hyperalgesic priming in mice.
Nat Commun. 15 (1): 1705. -
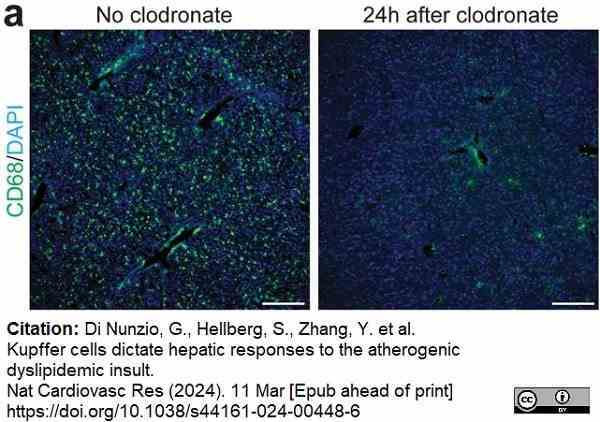
Di Nunzio, G. et al. (2024) Kupffer cells dictate hepatic responses to the atherogenic dyslipidemic insult
Nature Cardiovascular Research. 11 Mar [Epub ahead of print]. -
Lai, C. et al. (2022) Targeting inflammatory monocytes by immune-modifying nanoparticles prevents acute kidney allograft rejection.
Kidney Int. 102 (5): 1090-102. -
Kimura, H. et al. (2022) Purinergic P2X7 receptor antagonist ameliorates intestinal inflammation in postoperative ileus.
J Vet Med Sci. 84 (4): 610-7. -
Mohanta, S.K. et al. (2022) Neuroimmune cardiovascular interfaces control atherosclerosis.
Nature. 605 (7908): 152-9. -
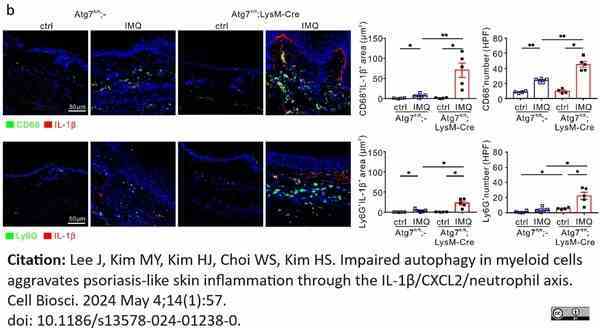
Lee, J. et al. (2024) Impaired autophagy in myeloid cells aggravates psoriasis-like skin inflammation through the IL-1β/CXCL2/neutrophil axis.
Cell Biosci. 14 (1): 57.
- Synonyms
- Macrosialin
- RRID
- AB_322219
- UniProt
- P31996
- Entrez Gene
- Cd68
- GO Terms
- GO:0005886 plasma membrane
- GO:0016021 integral to membrane
- GO:0005765 lysosomal membrane
- GO:0010008 endosome membrane
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Mouse ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up