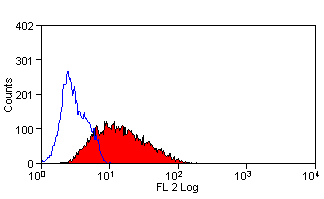
Leucoperm


Leucoperm
- Product Type
- Accessory Reagent
- Specificity
- Leucoperm
| Flow cytometric analyses with monoclonal antibodies have been restricted primarily to cell surface molecules. Intracellular structures such as cytoplasmic or nuclear enzymes, oncoproteins, cytokines, immunoglobulins etc. were largely excluded from such assays. Also excluded from flow cytometric assays were cytoplasmic localizations of well established membrane molecules such as CD3 and CD22. LEUCOPERM reagents allow intracellular antigen analysis with the same ease as surface antigens. The only prerequisite is the availability of suitable antibody conjugates. Most commercially available monoclonal antibody conjugates can be used with LEUCOPERM reagents. Some determinants are sensitive, however, to the fixation step involved. This and the optimal fixation time may have to be determined experimentally for each antibody conjugate. |
- Product Form
- Reagent A - Fixation medium
Reagent B - Permeabilisation medium - Preservative Stabilisers
- Formaldehyde in Reagent A
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Flow Cytometry |
- Instructions For Use
- For the detection of cell cycle antigens such as Ki-67, PCNA and BrdU, methanol modification is recommended - see protocol #F5.
1. Prepare cells in the appropriate manner. Adjust cell suspension to a concentration of 1 x 107 cells/ml in PBS/BSA. Whole blood samples may also be used. Bio-Rad recommend the use of EDTA anti-coagulant in these circumstances, although satisfactory results may be obtained using heparin or acid-citrate dextrose.
2. Add 100ul of cell suspension to the appropriate number of test tubes.
If required, perform staining of cell surface antigens at this stage. Following staining for the recommended period, wash cells once in PBS/BSA and discard supernatant.
3. Perform fixation of cells using appropriate fixation medium.
4. Add 3ml PBS/BSA and centrifuge for 5 minutes at 300 x g. Remove supernatant.
5. Re-suspend cells in 100ul of BUF09CB (Permeabilization Reagent).
6. Immediately add recommended volume of the appropriate directly conjugated antibody. Vortex and incubate for 30 minutes at room temperature.
If using an unconjugated primary antibody, wash in 3ml of PBS/BSA (as per step 5) and then repeat step 7 using an appropriate secondary antibody. There is no requirement to add further Leucoperm.
7. Incubate for 30 minutes at room temperature.
8. Wash cells with 3ml phosphate buffered saline and centrifuge for 5 minutes at 300 x g.
9. Wash once in PBS/BSA. Remove supernatant and re-suspend cells in sheath fluid for immediate analysis or re-suspend cells in 0.25ml of 0.5% formaldehyde and store them at 2-8oC in the dark. Analyse fixed cells within 24 hours. - For the detection of cell cycle antigens such as Ki-67, PCNA and BrdU, methanol modification is recommended - see protocol #F5.
1. Prepare cells in the appropriate manner. Adjust cell suspension to a concentration of 1 x 107 cells/ml in PBS/BSA. Whole blood samples may also be used. Bio-Rad recommend the use of EDTA anti-coagulant in these circumstances, although satisfactory results may be obtained using heparin or acid-citrate dextrose.
2. Add 100ul of cell suspension to the appropriate number of test tubes.
If required, perform staining of cell surface antigens at this stage. Following staining for the recommended period, wash cells once in PBS/BSA and discard supernatant.
3. Perform fixation of cells using appropriate fixation medium.
4. Add 3ml PBS/BSA and centrifuge for 5 minutes at 300 x g. Remove supernatant.
5. Re-suspend cells in 100ul of BUF09CB (Permeabilization Reagent).
6. Immediately add recommended volume of the appropriate directly conjugated antibody. Vortex and incubate for 30 minutes at room temperature.
If using an unconjugated primary antibody, wash in 3ml of PBS/BSA (as per step 5) and then repeat step 7 using an appropriate secondary antibody. There is no requirement to add further Leucoperm.
7. Incubate for 30 minutes at room temperature.
8. Wash cells with 3ml phosphate buffered saline and centrifuge for 5 minutes at 300 x g.
9. Wash once in PBS/BSA. Remove supernatant and re-suspend cells in sheath fluid for immediate analysis or re-suspend cells in 0.25ml of 0.5% formaldehyde and store them at 2-8oC in the dark. Analyse fixed cells within 24 hours. - For the detection of cell cycle antigens such as Ki-67, PCNA and BrdU, methanol modification is recommended - see protocol #F5.
1. Prepare cells in the appropriate manner. Adjust cell suspension to a concentration of 1 x 107 cells/ml in PBS/BSA. Whole blood samples may also be used. Bio-Rad recommend the use of EDTA anti-coagulant in these circumstances, although satisfactory results may be obtained using heparin or acid-citrate dextrose.
2. Add 100ul of cell suspension to the appropriate number of test tubes.
If required, perform staining of cell surface antigens at this stage. Following staining for the recommended period, wash cells once in PBS/BSA and discard supernatant.
3. Add 100ul of Reagent A (fixation medium, stored at room temperature).
4. Incubate for 15 minutes at room temperature.
5. Add 3ml PBS/BSA and centrifuge for 5 minutes at 300 x g. Remove supernatant.
6. Re-suspend cells in 100ul of Reagent B (Permeabilization Medium).
7. Immediately add recommended volume of the appropriate directly conjugated antibody. Vortex and incubate for 30 minutes at room temperature.
If using an unconjugated primary antibody, wash in 3ml of PBS/BSA (as per step 5) and then repeat step 7 using an appropriate secondary antibody. There is no requirement to add further Leucoperm.
8. Wash once in PBS/BSA. Remove supernatant and resuspend cells in sheath fluid for immediate analysis or resuspend cells in 0.25ml of 0.5% formaldehyde and store them at 2-8oC in the dark. Analyse fixed cells within 24 hours.
References for Leucoperm
-
Chiu, W.C. et al. (2009) Effects of dietary fish oil supplementation on cellular adhesion molecule expression and tissue myeloperoxidase activity in hypercholesterolemic mice with sepsis.
J Nutr Biochem. 20: 254-60. -
Grundy, M. et al. (2010) The FLT3 internal tandem duplication mutation is a secondary target of the aurora B kinase inhibitor AZD1152-HQPA in acute myelogenous leukemia cells.
Mol Cancer Ther. 9: 661-72. -
Taylor, L. et al. (2010) The effect of acute hypoxia on heat shock protein 72 expression and oxidative stress in vivo.
Eur J Appl Physiol. 109 (5): 849-55. -
Bairey, O. et al. (2010) Arsenic-trioxide-induced apoptosis of chronic lymphocytic leukemia cells.
Int J Lab Hematol. 32 (1 Pt 1): e77-85. -
Myles, A. et al. (2011) Expression of Toll-like receptors 2 and 4 is increased in peripheral blood and synovial fluid monocytes of patients with enthesitis-related arthritis subtype of juvenile idiopathic arthritis.
Rheumatology (Oxford). 50: 481-8. -
Osorio, Y. et al. (2011) Identification of small molecule lead compounds for visceral leishmaniasis using a novel ex vivo splenic explant model system
PLoS Negl Trop Dis. 5:e962. -
Suradhat, S. et al. (2015) A novel DNA vaccine for reduction of PRRSV-induced negative immunomodulatory effects: A proof of concept.
Vaccine. 33 (32): 3997-4003. -
Parry, D.A. et al. (2016) A homozygous STIM1 mutation impairs store-operated calcium entry and natural killer cell effector function without clinical immunodeficiency.
J Allergy Clin Immunol. 137 (3): 955-7.e8.
View The Latest Product References
-
Dishon, S. et al. (2017) Inhibition of Myeloid Differentiation Factor 88 Reduces Human and Mouse T-Cell Interleukin-17 and IFN&gamma Production and Ameliorates Experimental Autoimmune Encephalomyelitis Induced in Mice.
Front Immunol. 8: 615. -
Nie, H. et al. (2017) Phenotypic switch in lung interstitial macrophage polarization in an ovalbumin-induced mouse model of asthma.
Exp Ther Med. 14 (2): 1284-92. -
Jiang, W.J. et al. (2017) Structure-activity relationship of the inhibitory effects of flavonoids on nitric oxide production in RAW264.7 cells.
Bioorg Med Chem. 25 (2): 779-788. -
Kliminski, V. et al. (2017) Popdc1/Bves Functions in the Preservation of Cardiomyocyte Viability While Affecting Rac1 Activity and Bnip3 Expression.
J Cell Biochem. 118 (6): 1505-17. -
Arrieta-Villegas, C. et al. (2020) Immunogenicity and Protection against Mycobacterium caprae Challenge in Goats Vaccinated with BCG and Revaccinated after One Year.
Vaccines (Basel). 8 (4): 751. -
Alhuthali, H.M. et al. (2020) The natural alkaloid Jerantinine B has activity in acute myeloid leukemia cells through a mechanism involving c-Jun.
BMC Cancer. 20 (1): 629. -
Martelli, P. et al. (2021) Immune B cell responsiveness to single-dose intradermal vaccination against Mycoplasma hyopneumoniae..
Res Vet Sci. 141: 66-75. -
Hatzidaki, E. et al. (2021) A Novel Method for Colorectal Cancer Screening Based on Circulating Tumor Cells and Machine Learning.
Entropy (Basel). 23 (10): 1248. -
Martelli, P. et al. (2021) Immune B cell responsiveness to single-dose intradermal vaccination against Mycoplasma hyopneumoniae..
Res Vet Sci. 141: 66-75. -
Cequier, A. et al. (2022) Equine Mesenchymal Stem Cells Influence the Proliferative Response of Lymphocytes: Effect of Inflammation, Differentiation and MHC-Compatibility.
Animals (Basel). 12 (8) 984. -
Sanchez-Pino, M.D. (2022) Detection of Circulating and Tissue Myeloid-Derived Suppressor Cells (MDSC) by Flow Cytometry.
Methods Mol Biol. 2422: 247-61. -
Franzoni, G. et al. (2022) Analyses of the Impact of Immunosuppressive Cytokines on Porcine Macrophage Responses and Susceptibility to Infection to African Swine Fever Viruses.
Pathogens. 11 (2): 166. -
Jeong, E.M. et al. (2022) Targeting RUNX1 as a novel treatment modality for pulmonary arterial hypertension.
Cardiovasc Res. 118 (16): 3211-24. -
Matralis, D.T. et al. (2023) Intracellular IFN-γ and IL-4 levels of CD4 + and CD8 + T cells in the peripheral blood of naturally infected (Leishmania infantum) symptomatic dogs before and following a 4-week treatment with miltefosine and allopurinol: a double-blinded, controlled and cross-sectional study.
Acta Vet Scand. 65 (1): 2. -
Liu, Y. et al. (2024) Porous PLGA/MBG scaffold enhanced bone regeneration through osteoimmunomodulation
Composites Part B: Engineering. 272: 111202. -
Gordon, H. et al. (2024) Human Intestinal Dendritic Cells Can Overcome Retinoic Acid Signaling to Generate Proinflammatory CD4 T Cells with Both Gut and Skin Homing Properties.
J Immunol. 212 (1): 96-106.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
Always be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up