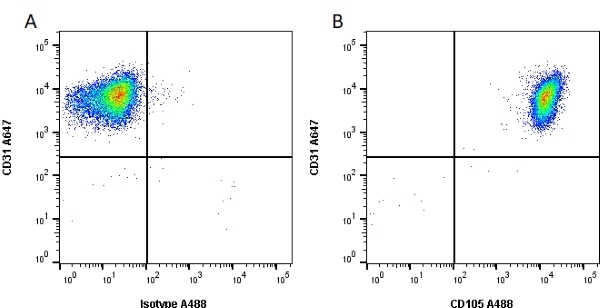
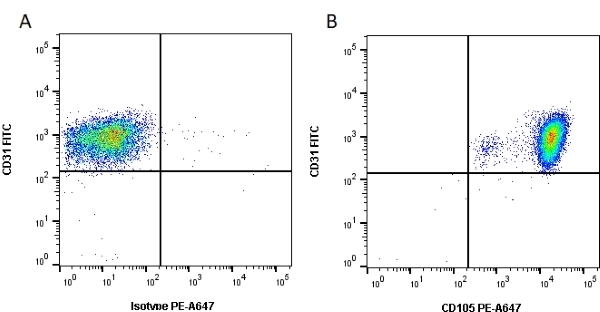
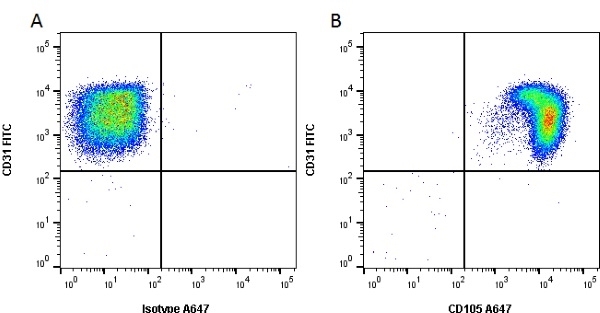
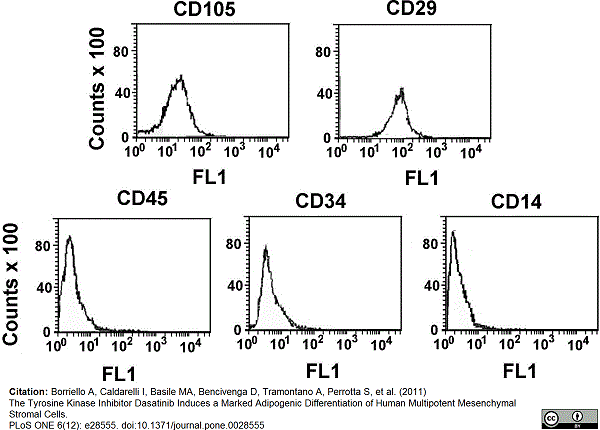
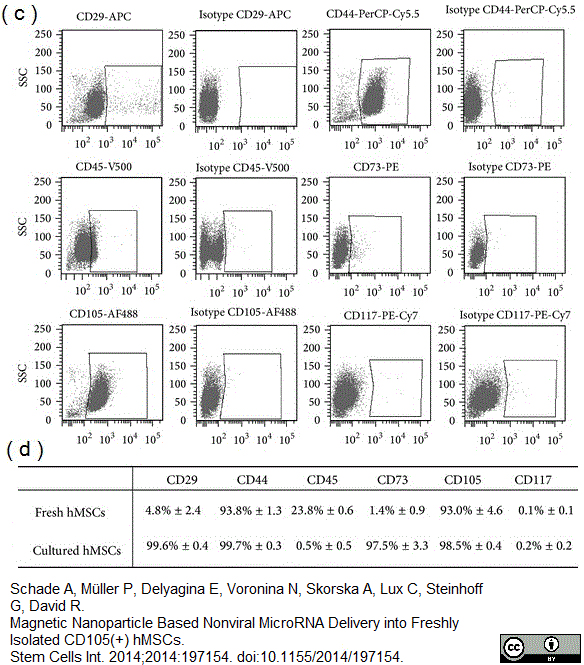
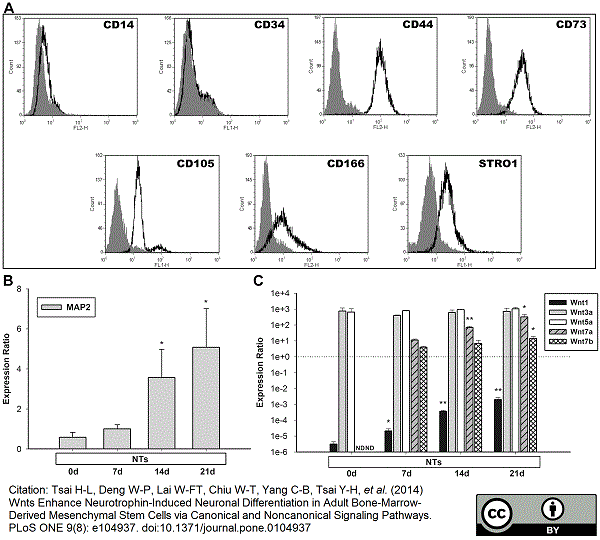
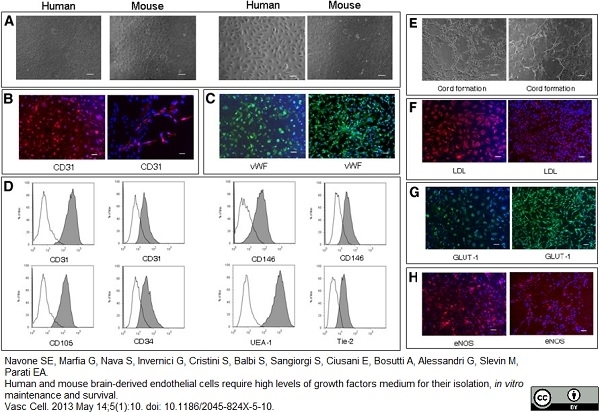
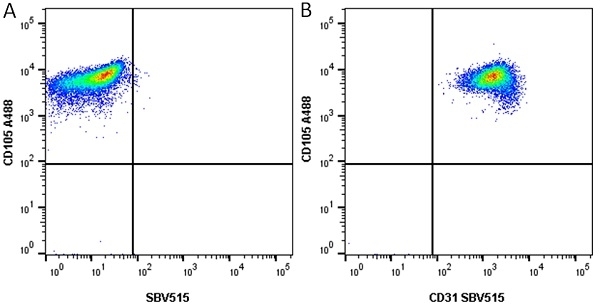
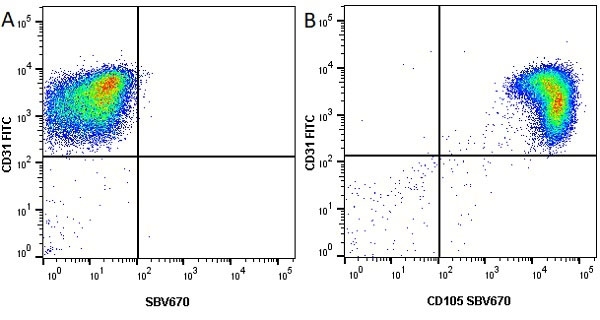
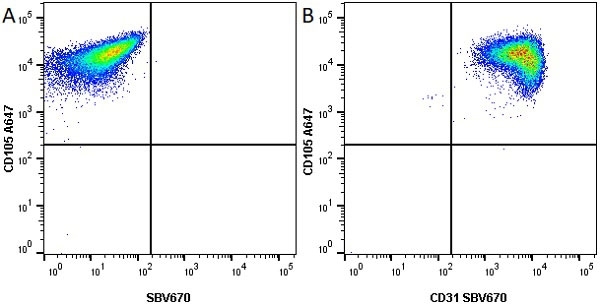
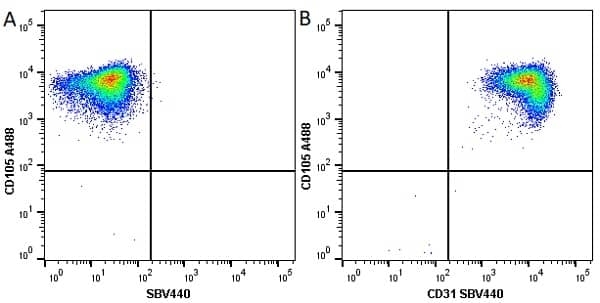
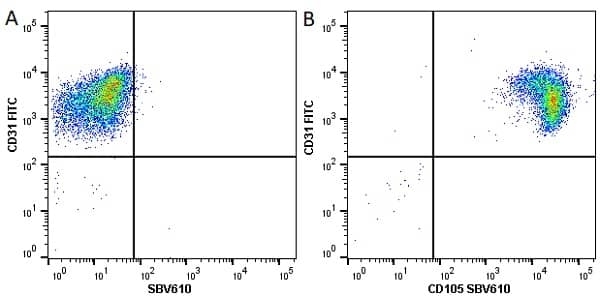
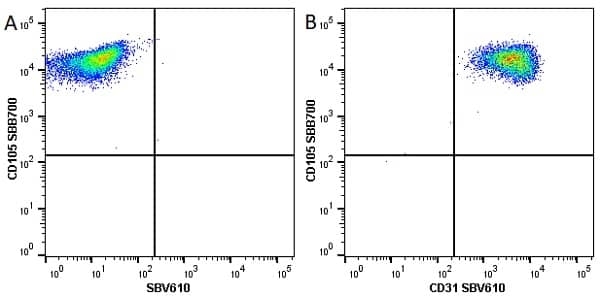
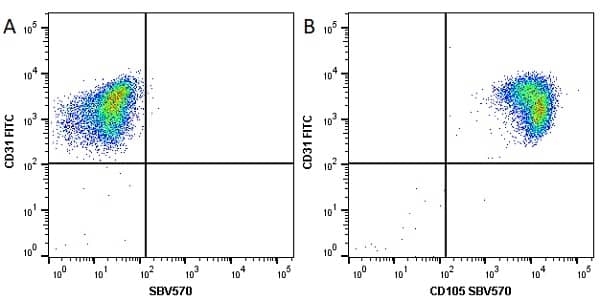
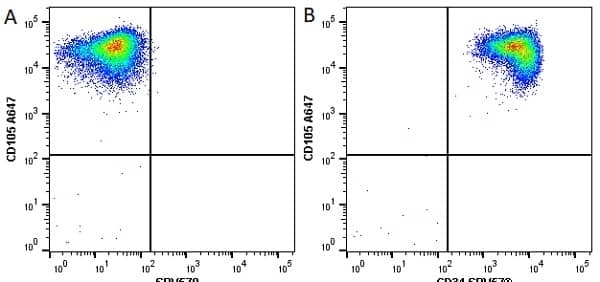
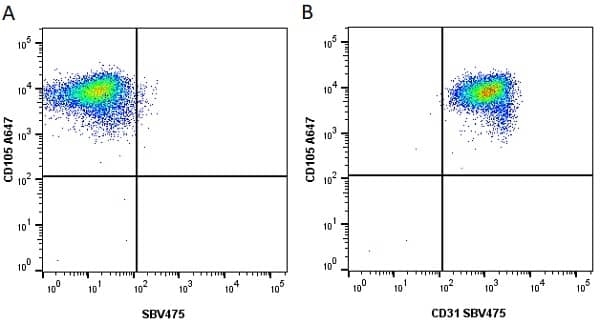
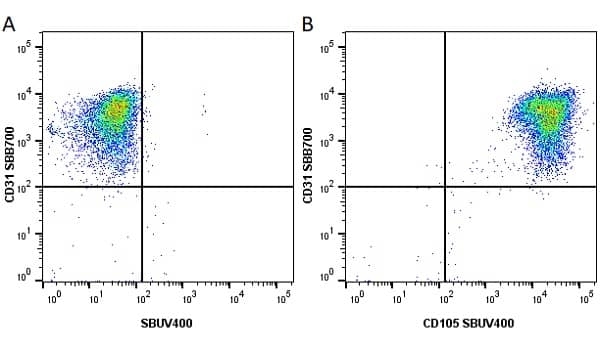
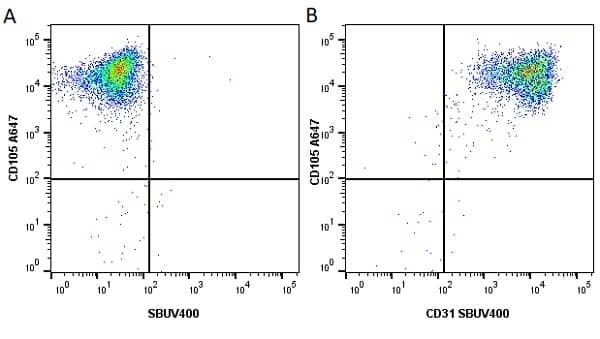
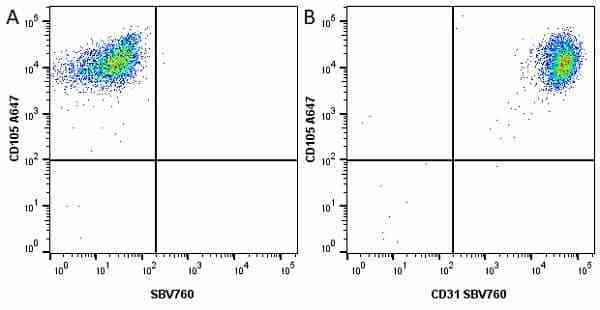
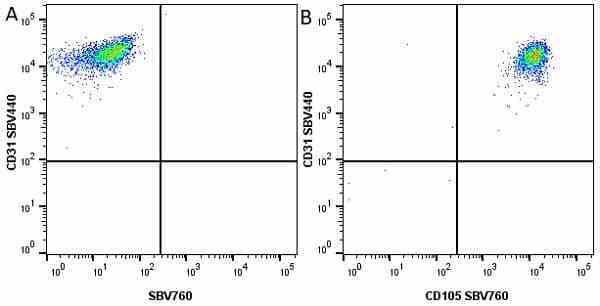
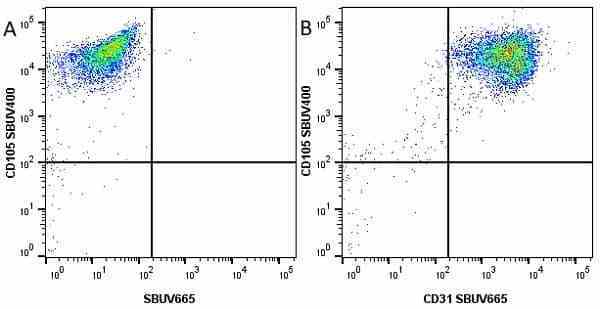
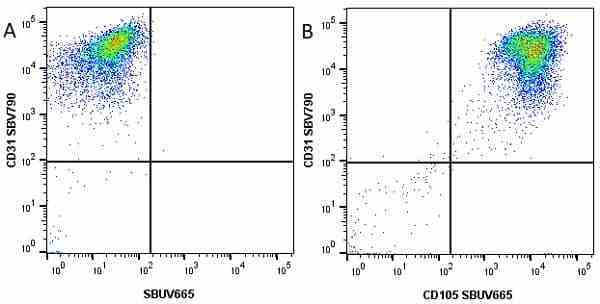
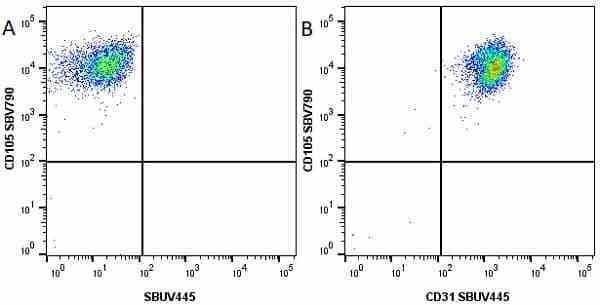
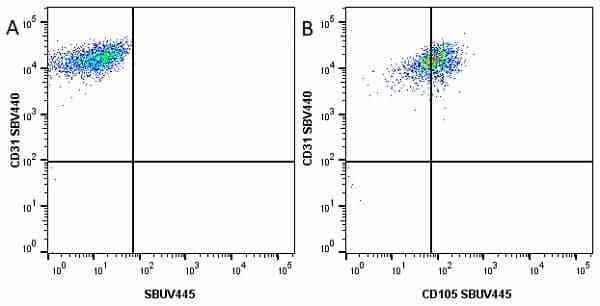
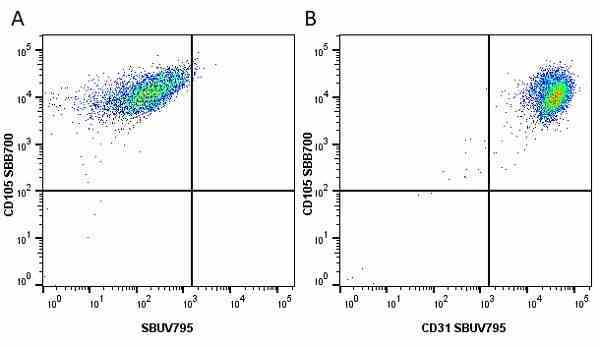
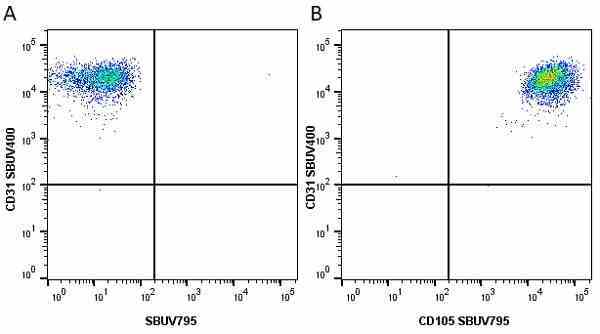
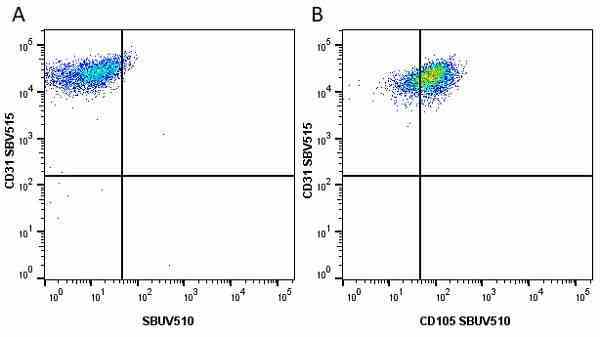
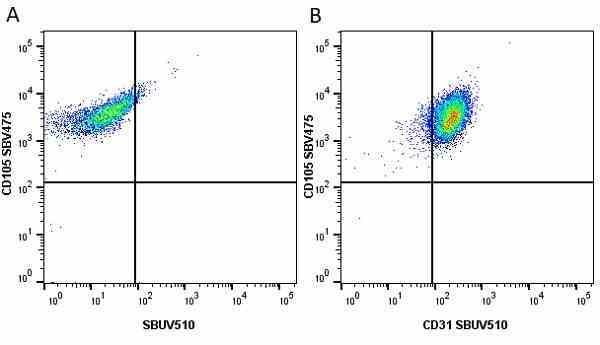
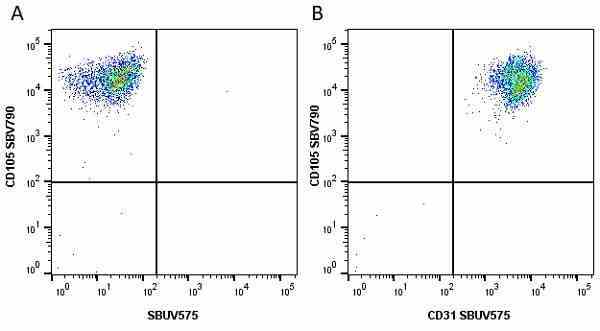
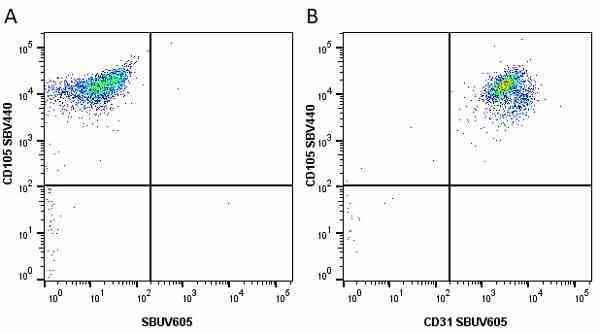
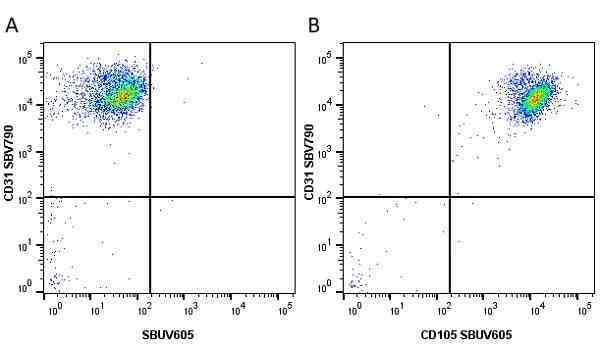
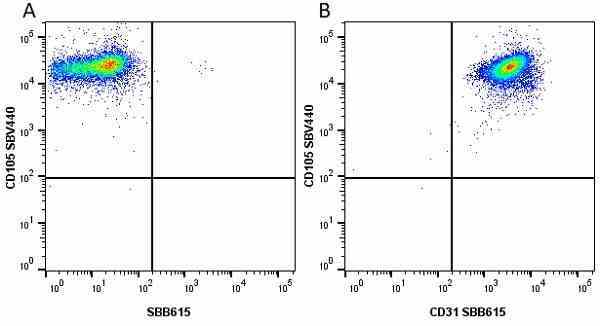
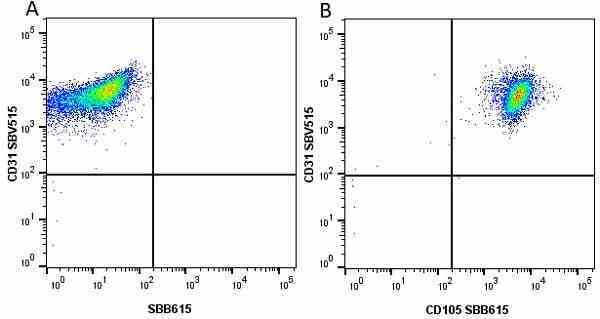
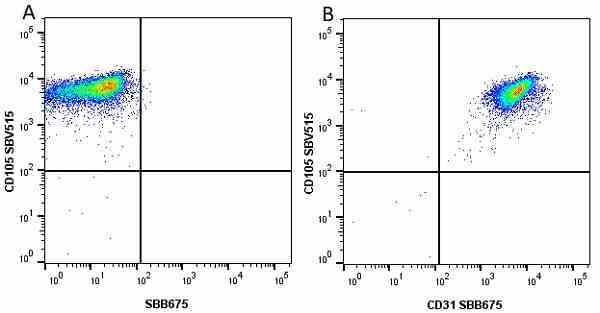
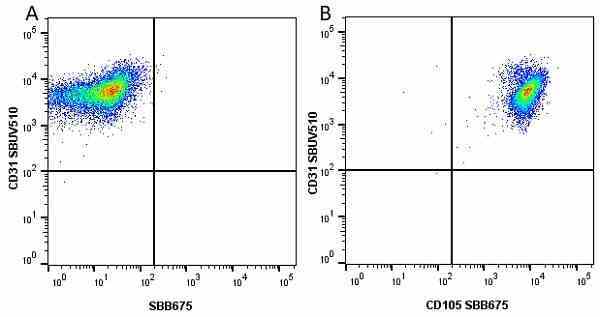
CD105 antibody | SN6




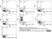


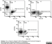
















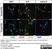







































Mouse anti Human CD105
- Product Type
- Monoclonal Antibody
- Clone
- SN6
- Isotype
- IgG1
- Specificity
- CD105
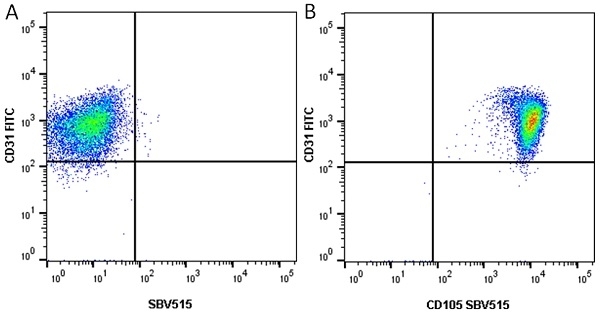
| Mouse anti Human CD105 antibody, clone SN6 recognizes human endoglin, also known as CD105. CD105 is a glycoprotein homodimer of ~95 kDa subunits expressed by endothelial cells, activated monocytes and some leukemia cells. |

Our CD105 (SN6) Antibody has been referenced in >73 publications* *Based on June 2020 data from CiteAb's antibody search engine. |
- Target Species
- Human
- Species Cross-Reactivity
-
Target Species Cross Reactivity Horse Cynomolgus monkey Rhesus Monkey Primate Expected from Sequence - N.B. Antibody reactivity and working conditions may vary between species.
- Product Form
- Purified IgG - liquid
- Preparation
- Purified IgG prepared by affinity chromatography on Protein G from tissue culture supernatant
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
- MCA1557: 0.09% sodium azide (NaN3)
-
MCA1557T: 0.09% sodium azide (NaN3)
1% bovine serum albumin - Carrier Free
- Yes
- Immunogen
- Partially purified cell membrane antigens from fresh leukemia cells
- Approx. Protein Concentrations
- IgG concentration 1.0 mg/ml
- Fusion Partners
- Spleen cells from immunized BALB/c mice were fused with cells of the mouse P3/NS1/1-Ag4-1 myeloma cell line
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Flow Cytometry | 1/10 | 1/50 | |
| Immunohistology - Frozen 1 | |||
| Immunohistology - Paraffin | |||
| Immunoprecipitation | |||
| Western Blotting |
- 1The epitope recognised by this antibody is reported to be sensitive to formaldehyde fixation and tissue processing. Bio-Rad recommends the use of acetone fixation for frozen sections.
- Flow Cytometry
- Use 10μl of the suggested working dilution to label 106 cells in 100μl
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Mouse IgG1 Negative Control | MCA928 | F | 100 Tests |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Mouse IgG1 Negative Control | ||||||
References for CD105 antibody
-
Haruta, Y. & Seon, B.K. (1986) Distinct human leukemia-associated cell surface glycoprotein GP160 defined by monoclonal antibody SN6.
Proc Natl Acad Sci USA 83 (20): 7898-902. -
Pierelli, L. et al. (2000) Modulation of bcl-2 and p27 in human primitive proliferating hematopoietic progenitors by autocrine TGF-beta1 is a cell cycle-independent effect and influences their hematopoietic potential.
Blood 95: 3001-9. -
Nagano, M. et al. (2007) Identification of functional endothelial progenitor cells suitable for the treatment of ischemic tissue using human umbilical cord blood.
Blood 110 (1): 151-60. -
Lozanoska-Ochser, B. et al. (2008) Expression of CD86 on human islet endothelial cells facilitates T cell adhesion and migration.
J Immunol. 181: 6109-16. -
Benetti, A. et al. (2008) Transforming growth factor-beta1 and CD105 promote the migration of hepatocellular carcinoma-derived endothelium.
Cancer Res. 68: 8626-34. -
Diaz-Romero, J. et al. (2008) Immunophenotypic changes of human articular chondrocytes during monolayer culture reflect bona fide dedifferentiation rather than amplification of progenitor cells.
J Cell Physiol. 214: 75-83. -
Sallustio, F. et al. (2010) TLR2 plays a role in the activation of human resident renal stem/progenitor cells.
FASEB J. 24: 514-25. -
Arufe, M.C. et al. (2010) Chondrogenic potential of subpopulations of cells expressing mesenchymal stem cell markers derived from human synovial membranes.
J Cell Biochem. 111: 834-45.
View The Latest Product References
-
Agha-Hosseini, F. et al. (2010) In vitro isolation of stem cells derived from human dental pulp.
Clin Transplant. 24: E23-8. -
Ferro, F. et al. (2010) Biochemical and biophysical analyses of tissue-engineered bone obtained from three-dimensional culture of a subset of bone marrow mesenchymal stem cells.
Tissue Eng Part A 16: 3657-67. -
Jin, H.J. et al. (2010) GD2 expression is closely associated with neuronal differentiation of human umbilical cord blood-derived mesenchymal stem cells.
Cell Mol Life Sci. 67 (11): 1845-58. -
Hauser, P.V. et al. (2010) Stem cells derived from human amniotic fluid contribute to acute kidney injury recovery.
Am J Pathol. 177: 2011-21. -
Braun, J. et al. (2010) Evaluation of the osteogenic and chondrogenic differentiation capacities of equine adipose tissue-derived mesenchymal stem cells.
Am J Vet Res. 71 (10): 1228-36. -
Balmayor, E.R. et al. (2011) Synthesis and functionalization of superparamagnetic poly-ε-caprolactone microparticles for the selective isolation of subpopulations of human adipose-derived stem cells.
J R Soc Interface 8: 896-908. -
Ciccocioppo, R. et al. (2011) Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease.
Gut 60: 788-98. -
Cox, G. et al. (2011) The use of the reamer-irrigator-aspirator to harvest mesenchymal stem cells.
J Bone Joint Surg Br. 93: 517-24. -
De Schauwer, C. et al. (2012) In search for cross-reactivity to immunophenotype equine mesenchymal stromal cells by multicolor flow cytometry.
Cytometry A 81: 312-23. -
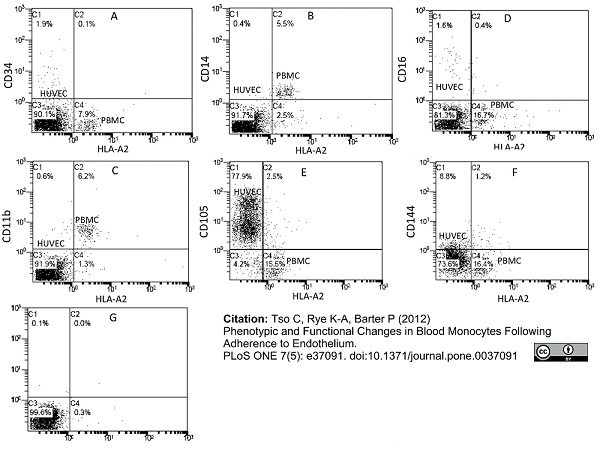
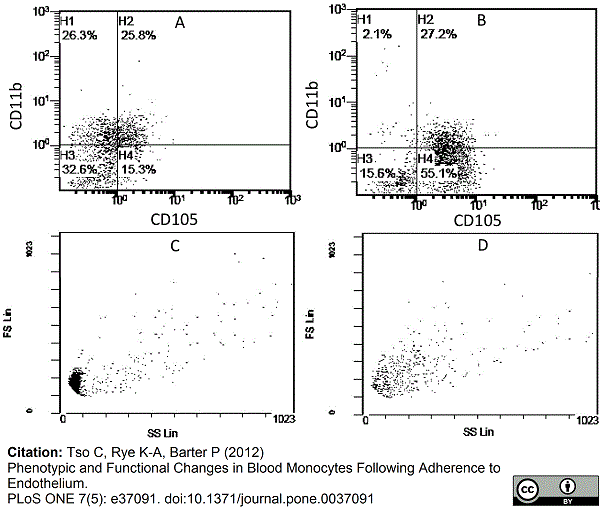
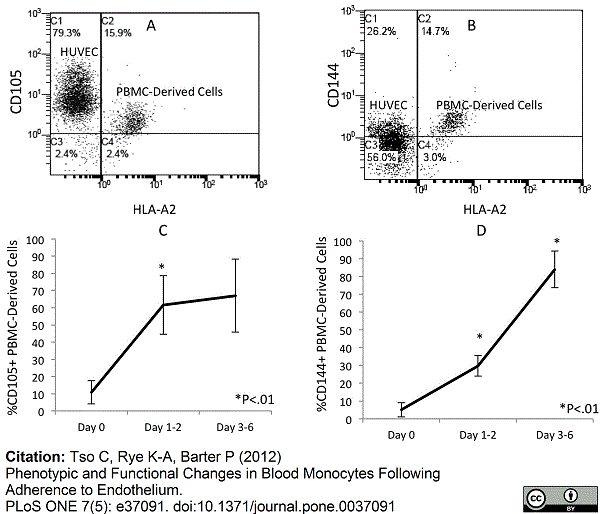
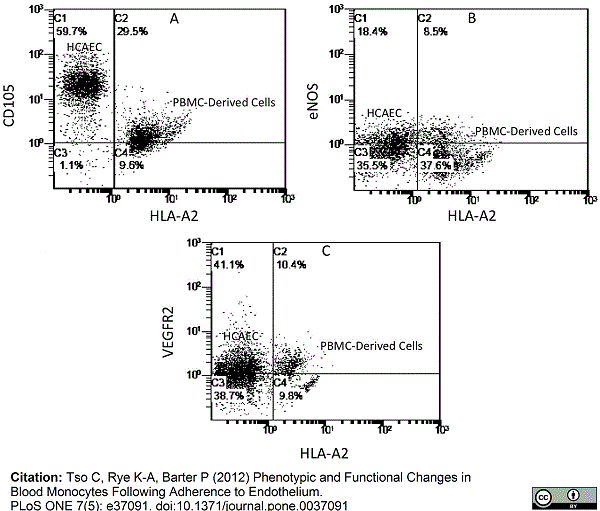
Tso, C. et al. (2012) Phenotypic and functional changes in blood monocytes following adherence to endothelium.
PLoS One 7: e37091. -
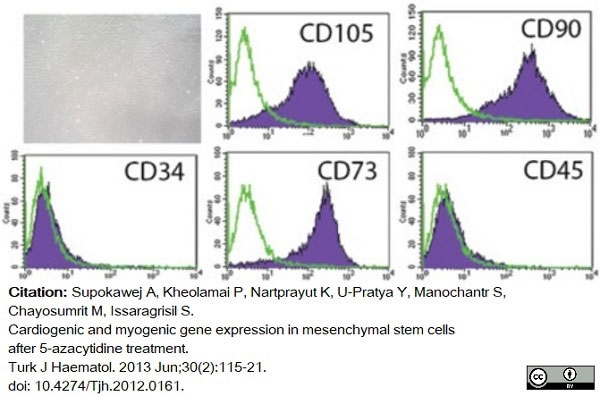
Supokawej, A. et al. (2013) Cardiogenic and myogenic gene expression in mesenchymal stem cells after 5-azacytidine treatment.
Turk J Haematol. 30 (2): 115-21. -
Mehrkens, A. et al. (2013) Non-adherent mesenchymal progenitors from adipose tissue stromal vascular fraction.
Tissue Eng Part A 20: 1081-8. -
Kang, S.D. et al. (2013) Isolation of Functional Human Endothelial Cells from Small Volumes of Umbilical Cord Blood.
Ann Biomed Eng. 41: 2181-92. -
Cho, H.J. et al. (2013) Generation of human secondary cardiospheres as a potent cell processing strategy for cell-based cardiac repair.
Biomaterials 34: 651-61. -
Hu, N. et al. (2013) Long-term outcome of the repair of 50 mm long median nerve defects in rhesus monkeys with marrow mesenchymal stem cells-containing, chitosan-based tissue engineered nerve grafts.
Biomaterials 34: 100-11. -
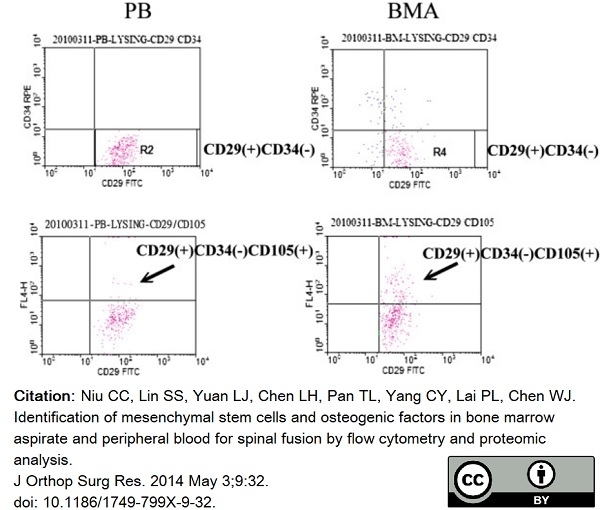
Niu, C.C. et al. (2014) Identification of mesenchymal stem cells and osteogenic factors in bone marrow aspirate and peripheral blood for spinal fusion by flow cytometry and proteomic analysis.
J Orthop Surg Res. 9: 32. -
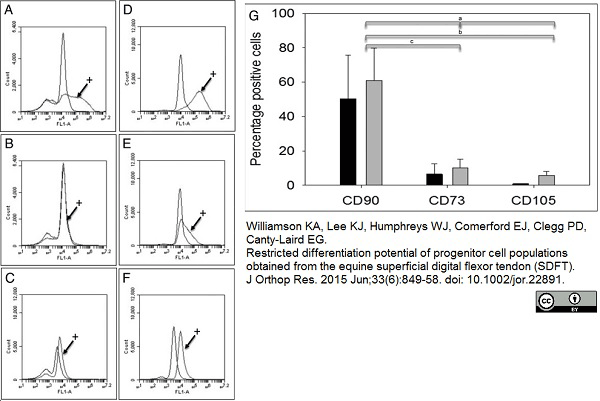
Williamson, K.A. et al. (2015) Restricted differentiation potential of progenitor cell populations obtained from the equine superficial digital flexor tendon (SDFT).
J Orthop Res. 33 (6): 849-58. -
Yi, T. et al. (2015) Manufacture of Clinical-Grade Human Clonal Mesenchymal Stem Cell Products from Single Colony Forming Unit-Derived Colonies Based on the Subfractionation Culturing Method.
Tissue Eng Part C Methods. 21 (12): 1251-62. -
Mumaw, J.L. et al. (2015) Feline mesenchymal stem cells and supernatant inhibit reactive oxygen species production in cultured feline neutrophils.
Res Vet Sci. 103: 60-9. -
Zhang, J. et al. (2016) Bone mesenchymal stem cells differentiate into myofibroblasts in the tumor microenvironment.
Oncol Lett. 12 (1): 644-50. -
Morsing, M. et al. (2016) Evidence of two distinct functionally specialized fibroblast lineages in breast stroma.
Breast Cancer Res. 18 (1): 108. -
Boccardo, S. et al. (2016) Engineered mesenchymal cell-based patches as controlled VEGF delivery systems to induce extrinsic angiogenesis.
Acta Biomater. 42: 127-35. -
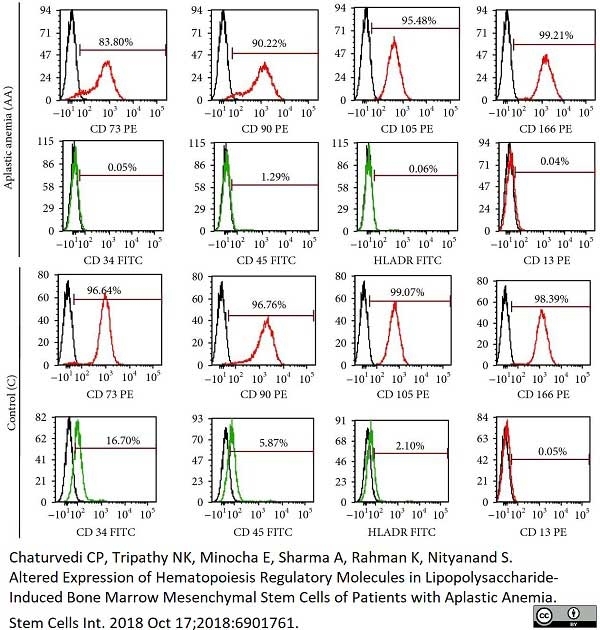
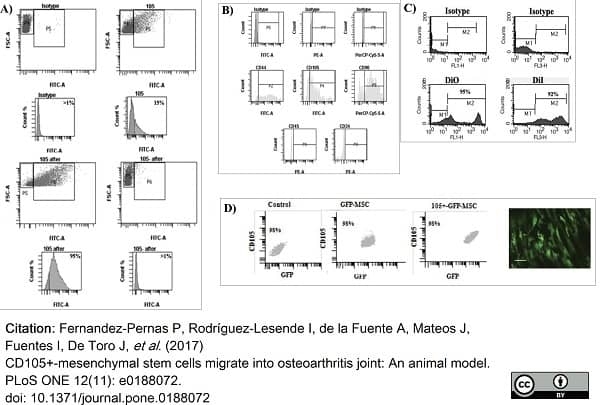
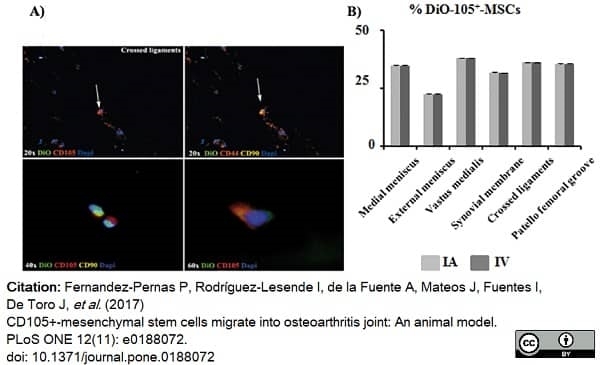
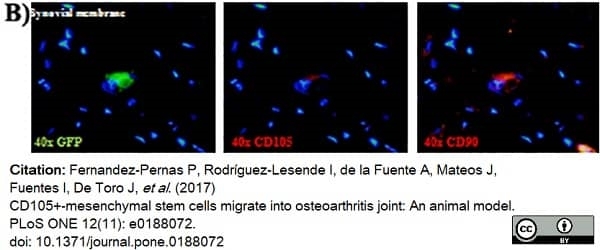
Fernandez-Pernas, P. et al. (2017) CD105+-mesenchymal stem cells migrate into osteoarthritis joint: An animal model.
PLoS One. 12 (11): e0188072. -
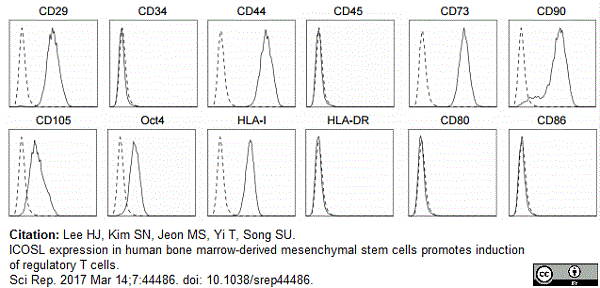
Lee, H.J. et al. (2017) ICOSL expression in human bone marrow-derived mesenchymal stem cells promotes induction of regulatory T cells.
Sci Rep. 7: 44486. -
Bertolo, A. et al. (2017) Oxidative status predicts quality in human mesenchymal stem cells.
Stem Cell Res Ther. 8 (1): 3. -
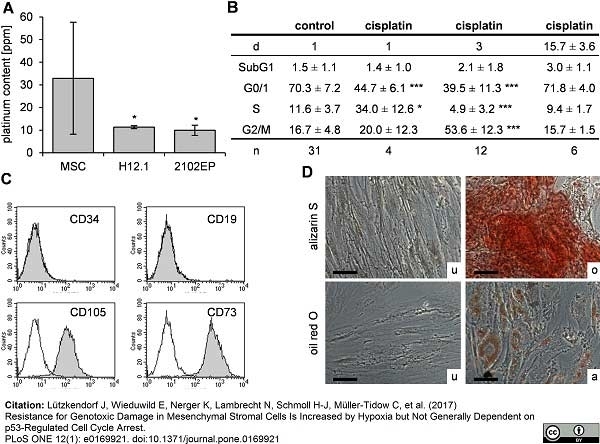
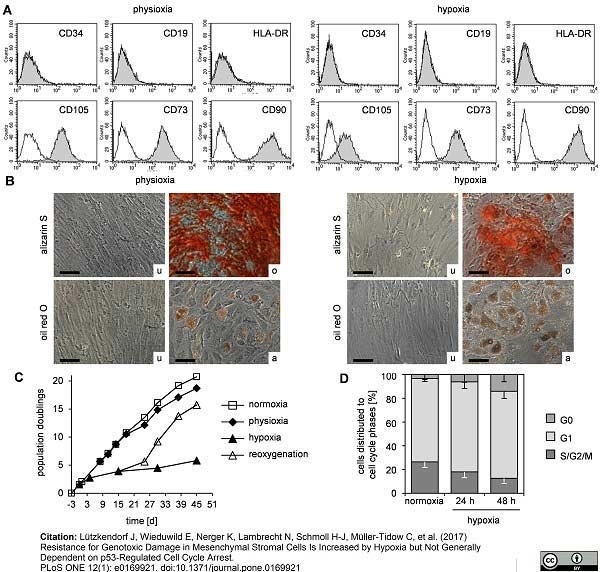
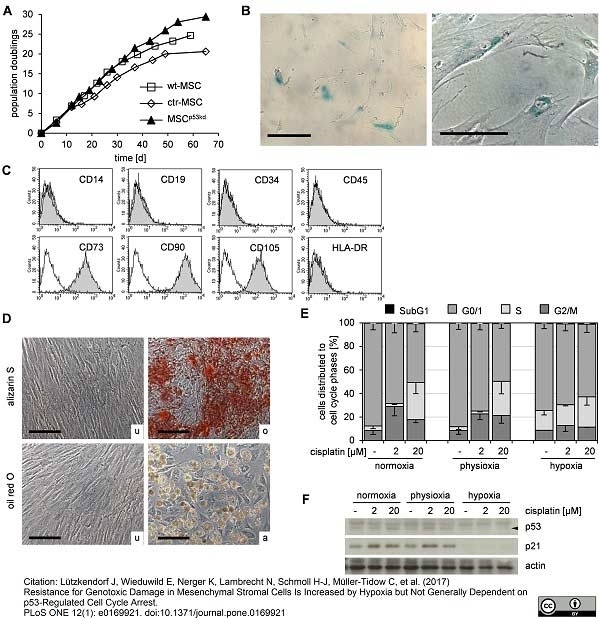
Lützkendorf, J. et al. (2017) Resistance for Genotoxic Damage in Mesenchymal Stromal Cells Is Increased by Hypoxia but Not Generally Dependent on p53-Regulated Cell Cycle Arrest.
PLoS One. 12 (1): e0169921. -
Santos,V.H.D. et al. (2019) Evaluation of alginate hydrogel encapsulated mesenchymal stem cell migration in horses.
Res Vet Sci. 124: 38-45. -
GarikipatiV, N.S. et al. (2018) Isolation and characterization of mesenchymal stem cells from human fetus heart.
PLoS One. 13 (2): e0192244. -
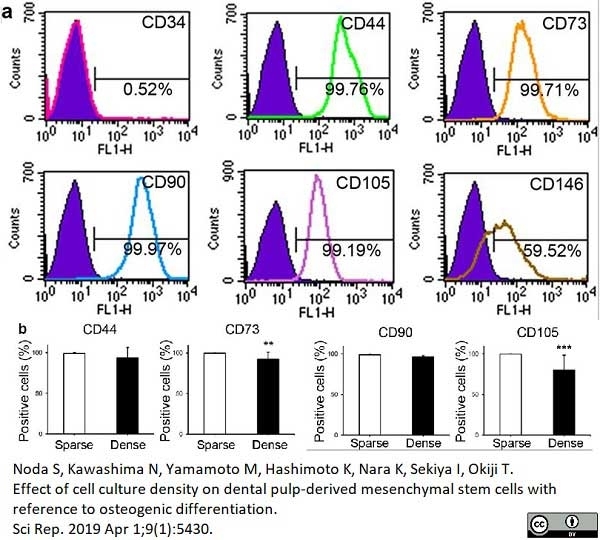
Noda, S. et al. (2019) Effect of cell culture density on dental pulp-derived mesenchymal stem cells with reference to osteogenic differentiation.
Sci Rep. 9 (1): 5430. -
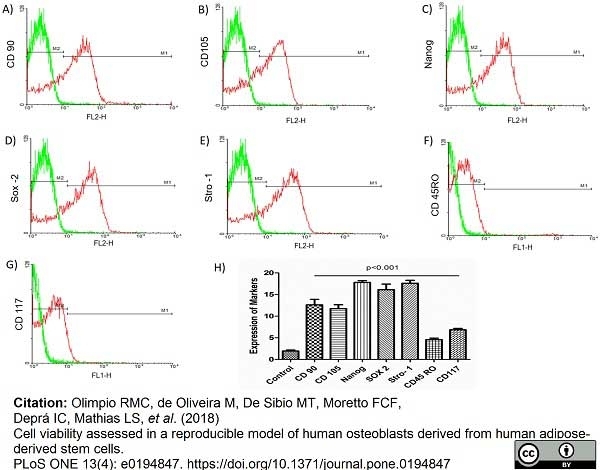
Olimpio, R.M.C. et al. (2018) Cell viability assessed in a reproducible model of human osteoblasts derived from human adipose-derived stem cells.
PLoS One. 13 (4): e0194847. -
Lotfi, R. et al. (2018) ATP promotes immunosuppressive capacities of mesenchymal stromal cells by enhancing the expression of indoleamine dioxygenase.
Immun Inflamm Dis. 6 (4): 448-55. -
May, J.E. et al. (2018) Chemotherapy-induced genotoxic damage to bone marrow cells: long-term implications.
Mutagenesis. 33 (3): 241-251. -
Piñeiro-Ramil, M. et al. (2020) Immortalizing Mesenchymal Stromal Cells from Aged Donors While Keeping Their Essential Features.
Stem Cells Int. 2020: 5726947. -
Rey, F. et al. (2019) Adipose-Derived Stem Cells from Fat Tissue of Breast Cancer Microenvironment Present Altered Adipogenic Differentiation Capabilities.
Stem Cells Int. 2019: 1480314. -
Kim, M. et al. (2020) A Small-Sized Population of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Shows High Stemness Properties and Therapeutic Benefit.
Stem Cells Int. 2020: 5924983. -
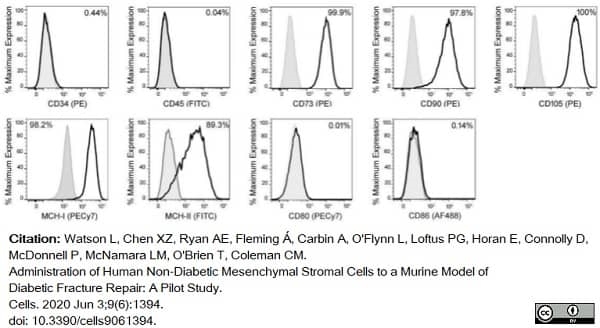
Watson, L. et al. (2020) Administration of Human Non-Diabetic Mesenchymal Stromal Cells to a Murine Model of Diabetic Fracture Repair: A Pilot Study.
Cells. 9 (6): 1394. -
Cargnoni, A. et al. (2020) Amniotic MSCs reduce pulmonary fibrosis by hampering lung B-cell recruitment, retention, and maturation.
Stem Cells Transl Med. 9 (9): 1023-35. -
Manini, I. et al. (2020) Heterogeneity Matters: Different Regions of Glioblastoma Are Characterized by Distinctive Tumor-Supporting Pathways.
Cancers (Basel). 12 (10): 2960. -
Kim, S.H. et al. (2019) Forkhead box O1 (FOXO1) controls the migratory response of Toll-like receptor (TLR3)-stimulated human mesenchymal stromal cells.
J Biol Chem. 294 (21): 8424-37. -
Lotfi, R. et al. (2020) Validation of Microbiological Testing of Cellular Medicinal Products Containing Antibiotics.
Transfus Med Hemother. 47 (2): 144-51. -
Di Paola, A. et al. (2021) Eltrombopag in paediatric immune thrombocytopenia: Iron metabolism modulation in mesenchymal stromal cells.
Br J Haematol. 97 (1): 110-119. -
Piñeiro-Ramil, M. et al. (2021) Generation of Mesenchymal Cell Lines Derived from Aged Donors.
Int J Mol Sci. 22 (19): 10667. -
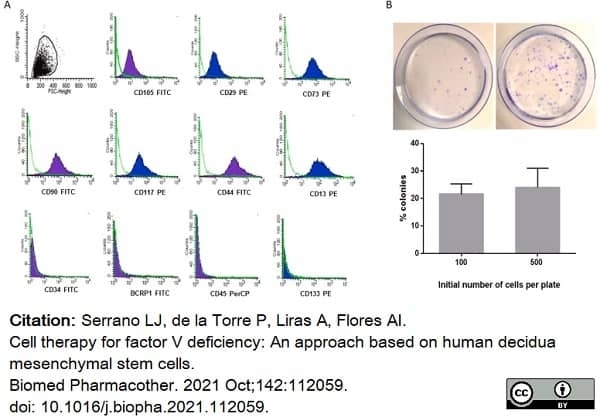
Serrano, L.J. et al. (2021) Cell therapy for factor V deficiency: An approach based on human decidua mesenchymal stem cells.
Biomed Pharmacother. 142: 112059. -
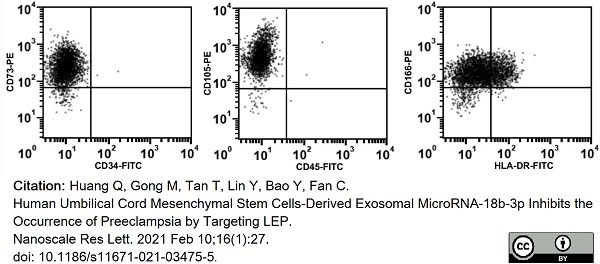
Huang, Q. et al. (2021) Human Umbilical Cord Mesenchymal Stem Cells-Derived Exosomal MicroRNA-18b-3p Inhibits the Occurrence of Preeclampsia by Targeting LEP.
Nanoscale Res Lett. 16 (1): 27. -
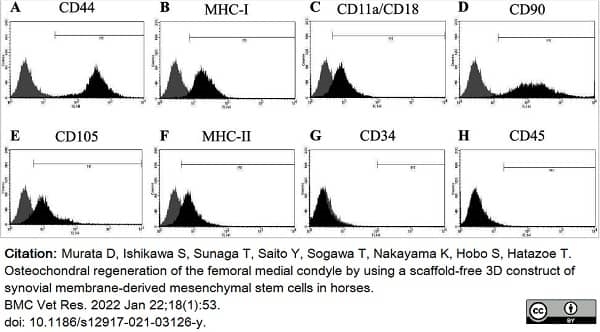
Murata, D. et al. (2022) Osteochondral regeneration of the femoral medial condyle by using a scaffold-free 3D construct of synovial membrane-derived mesenchymal stem cells in horses.
BMC Vet Res. 18 (1): 53. -
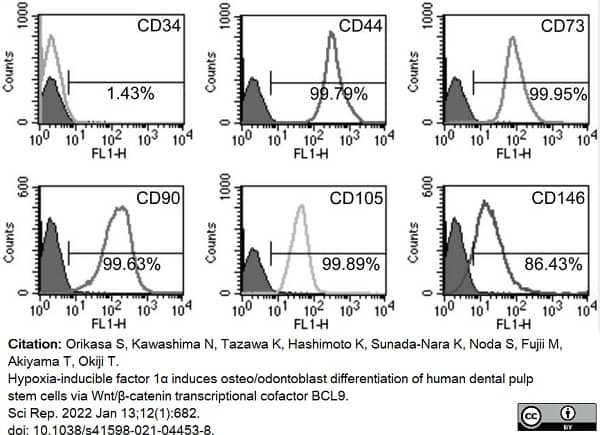
Orikasa, S. et al. (2022) Hypoxia-inducible factor 1α induces osteo/odontoblast differentiation of human dental pulp stem cells via Wnt/β-catenin transcriptional cofactor BCL9.
Sci Rep. 12 (1): 682. -
Freitag, N. et al. (2022) Eutopic endometrial immune profile of infertility-patients with and without endometriosis.
J Reprod Immunol. 150: 103489. -
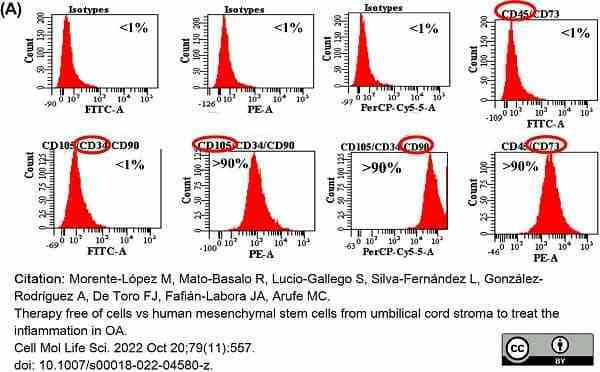
Morente-López, M. et al. (2022) Therapy free of cells vs human mesenchymal stem cells from umbilical cord stroma to treat the inflammation in OA.
Cell Mol Life Sci. 79 (11): 557. -
Creamer, D.G. et al. (2022) Influence of exposure to microbial ligands, immunosuppressive drugs and chronic kidney disease on endogenous immunomodulatory gene expression in feline adipose-derived mesenchymal stem cells.
J Feline Med Surg. 24 (6): e43-e56. -
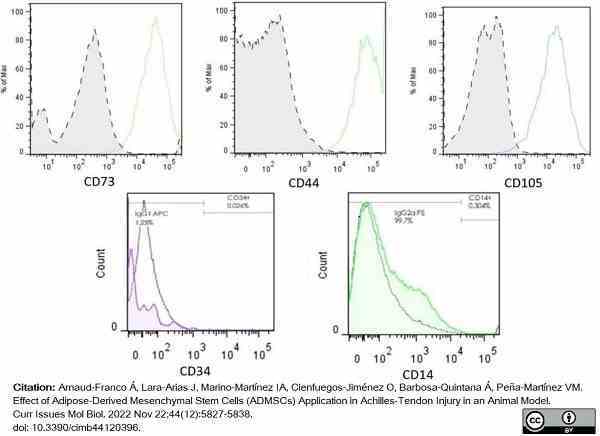
Arnaud-Franco, Á. et al. (2022) Effect of Adipose-Derived Mesenchymal Stem Cells (ADMSCs) Application in Achilles-Tendon Injury in an Animal Model.
Curr Issues Mol Biol. 44 (12): 5827-38. -
Piñeiro-Ramil, M. et al. (2023) Generation of human immortalized chondrocytes from osteoarthritic and healthy cartilage : a new tool for cartilage pathophysiology studies.
Bone Joint Res. 12 (1): 46-57. -
Connolly, D.M. et al. (2023) Early Human Pathophysiological Responses to Exertional Hypobaric Decompression Stress.
Aerosp Med Hum Perform. 94 (10): 738-49. -
Jakl, V. et al. (2023) Effect of Expansion Media on Functional Characteristics of Bone Marrow-Derived Mesenchymal Stromal Cells.
Cells. 12 (16): 2105. -
Tafuri, W.L. et al. (2022) Skin fibrosis associated with keloid, scleroderma and Jorge Lobo's disease (lacaziosis): An immuno-histochemical study.
Int J Exp Pathol. 103 (6): 234-44. -
Tiraihi, T. et al. (2023) A Sequential Culturing System for Generating Epithelial-Like Stem Cells from Human Mesenchymal Stem Cells Derived from Adipose Tissue
Cell and Tissue Biology. 17 (6): 639-52. -
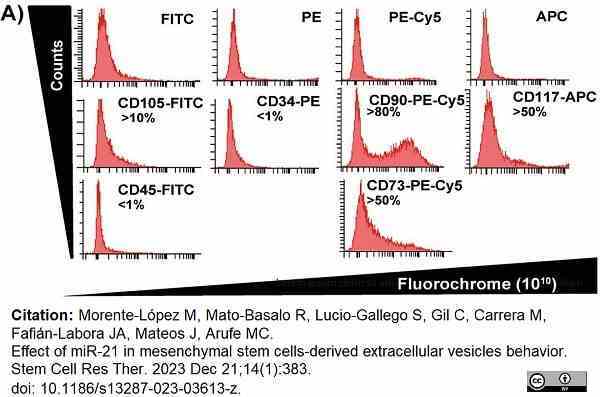
Morente-López, M. et al. (2023) Effect of miR-21 in mesenchymal stem cells-derived extracellular vesicles behavior.
Stem Cell Res Ther. 14 (1): 383. -
Tripathy, N.K. et al. (2018) Cardiomyogenic Heterogeneity of Clonal Subpopulations of Human Bone Marrow Mesenchymal Stem Cells.
J Stem Cells Regen Med. 14 (1): 27-33. -
Karpyuk, V. et al. (2019) Innovation-based Approach in Reconstruction of Reduced Jaw Alveolar Ridge Bone using Cell Regeneration Technologies
Archiv Euromedica 9 (2): 147-55.
Further Reading
-
Carrade, D.D. et al. (2012) Comparative Analysis of the Immunomodulatory Properties of Equine Adult-Derived Mesenchymal Stem Cells.
Cell Med. 4: 1-11. -
Burk, J. et al. (2013) Equine cellular therapy--from stall to bench to bedside?
Cytometry A 83 (1): 103-13.
- Synonyms
- Endoglin
- RRID
- AB_321986
- UniProt
- P17813
- Entrez Gene
- ENG
- GO Terms
- GO:0001300 chronological cell aging
- GO:0001569 patterning of blood vessels
- GO:0001937 negative regulation of endothelial cell proliferation
- GO:0001947 heart looping
- GO:0003084 positive regulation of systemic arterial blood pressure
- GO:0007155 cell adhesion
- GO:0004888 transmembrane receptor activity
- GO:0005024 transforming growth factor beta receptor activity
- GO:0005072 transforming growth factor beta receptor, cytoplasmic mediator activity
- View More GO Terms
- GO:0005114 type II transforming growth factor beta receptor binding
- GO:0005534 galactose binding
- GO:0005539 glycosaminoglycan binding
- GO:0005615 extracellular space
- GO:0005624 membrane fraction
- GO:0007179 transforming growth factor beta receptor signaling pathway
- GO:0050431 transforming growth factor beta binding
- GO:0009897 external side of plasma membrane
- GO:0009986 cell surface
- GO:0010552 positive regulation of gene-specific transcription from RNA polymerase II promoter
- GO:0010553 negative regulation of gene-specific transcription from RNA polymerase II promoter
- GO:0010862 positive regulation of pathway-restricted SMAD protein phosphorylation
- GO:0017015 regulation of transforming growth factor beta receptor signaling pathway
- GO:0022009 central nervous system vasculogenesis
- GO:0022617 extracellular matrix disassembly
- GO:0030155 regulation of cell adhesion
- GO:0030509 BMP signaling pathway
- GO:0030512 negative regulation of transforming growth factor beta receptor signaling pathway
- GO:0030513 positive regulation of BMP signaling pathway
- GO:0031953 negative regulation of protein autophosphorylation
- GO:0034713 type I transforming growth factor beta receptor binding
- GO:0042060 wound healing
- GO:0042127 regulation of cell proliferation
- GO:0042803 protein homodimerization activity
- GO:0045449 regulation of transcription
- GO:0048185 activin binding
- GO:0048745 smooth muscle tissue development
- GO:0048844 artery morphogenesis
- GO:0048845 venous blood vessel morphogenesis
- GO:0051001 negative regulation of nitric-oxide synthase activity
- GO:0060326 cell chemotaxis
- GO:0060394 negative regulation of pathway-restricted SMAD protein phosphorylation
- GO:0070022 transforming growth factor beta receptor complex
- GO:0070483 detection of hypoxia
MCA1557
MCA1557T
If you cannot find the batch/lot you are looking for please contact our technical support team for assistance.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Human ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up