Myelin Proteolipid Protein antibody | plpc1



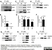


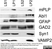


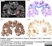

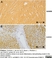

















Mouse anti Myelin Proteolipid Protein
- Product Type
- Monoclonal Antibody
- Clone
- plpc1
- Isotype
- IgG2a
- Specificity
- Myelin Proteolipid Protein
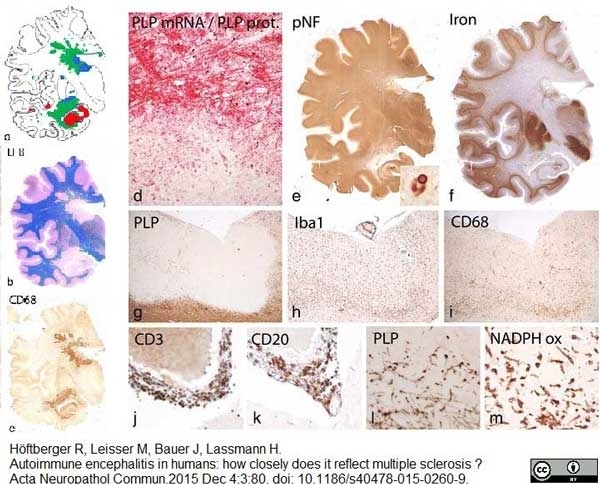
| Mouse anti myelin proteolipid protein antibody, clone plpc1 recognizes myelin proteolipid protein (PLP) in many mammalian species (Stoffel et al. 1985). Clone plpc1 also recognizes the alternative PLP splice variant lacking part of the cytoplasmic domain (amino acids 117-151), known as DM20 (Simons et al. 1987) . PLP encodes the major protein components of compact CNS myelin and mutations in the PLP gene can lead to severe dysmyelinating disease (Hudson et al. 1989). Mouse anti myelin proteolipid protein, clone plpc1 has proved a useful immunohistochemical tool for the study of central nervous system injury in patients with multiple sclerosis (Seewan et al. 2011, Huizinga et al. 2011) |
- Target Species
- Bovine
- Species Cross-Reactivity
-
Target Species Cross Reactivity Human Tenerife lizard (Gallotia galloti) - N.B. Antibody reactivity and working conditions may vary between species.
- Product Form
- Purified IgG - liquid
- Preparation
- Purified IgG prepared by affinity chromatography on Protein G from tissue culture supernatant
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
- <0.1% Sodium Azide (NaN3)
- Immunogen
- Synthetic peptide GRGTKF corresponding to C terminal region of myelin proteolipid protein.
- Approx. Protein Concentrations
- IgG concentration 1 mg/ml
- Fusion Partners
- Spleen cells from immunized BALB/c mice were fused with cells of the mouse SP2/0 myeloma cell line.
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Flow Cytometry | |||
| Immunofluorescence | |||
| Immunohistology - Frozen | |||
| Immunohistology - Paraffin | |||
| Western Blotting |
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Mouse IgG2a Negative Control | MCA929 | F | 100 Tests |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Mouse IgG2a Negative Control | ||||||
References for Myelin Proteolipid Protein antibody
-
Boon, L. et al. (2001) Prevention of experimental autoimmune encephalomyelitis in the common marmoset (Callithrix jacchus) using a chimeric antagonist monoclonal antibody against human CD40 is associated with altered B cell responses.
J Immunol. 167: 2942-9. -
Jaśkiewicz, E. et al. (2005) Expression of recombinant forms of human 21.5 kDa myelin basic protein and proteolipid protein in CHO cells.
Acta. Biochim. Pol. 52: 863-6. -
Pomeroy, I.M. et al. (2005) Demyelinated neocortical lesions in marmoset autoimmune encephalomyelitis mimic those in multiple sclerosis.
Brain. 128: 2713-21. -
Jatana, M. et al. (2006) Combination of systemic hypothermia and N-acetylcysteine attenuates hypoxic-ischemic brain injury in neonatal rats.
Pediatr Res. 59 (5): 684-9. -
Santos, E. et al. (2006) Peculiar and typical oligodendrocytes are involved in an uneven myelination pattern during the ontogeny of the lizard visual pathway.
J Neurobiol. 66 (10): 1115-24. -
Gilmore, C.P. et al. (2006) Spinal cord gray matter demyelination in multiple sclerosis-a novel pattern of residual plaque morphology.
Brain Pathol. 16: 202-8. -
van Horssen, J. et al. (2006) NAD(P)H:quinone oxidoreductase 1 expression in multiple sclerosis lesions.
Free Radic Biol Med. 41: 311-7. -
Roemer, S.F. et al. (2007) Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis.
Brain. 130: 1194-205.
View The Latest Product References
-
Geurts, J.J. et al. (2007) Extensive hippocampal demyelination in multiple sclerosis.
J Neuropathol Exp Neurol. 66: 819-27. -
Moharregh-Khiabani, D. et al. (2010) Effects of fumaric acids on cuprizone induced central nervous system de- and remyelination in the mouse.
PLoS One. 5:e11769. -
van Horssen, J. et al. (2010) Nrf2 and DJ1 are consistently upregulated in inflammatory multiple sclerosis lesions.
Free Radic Biol Med. 49: 1283-9. -
Popescu, B.F. et al. (2010) Absence of cortical demyelination in neuromyelitis optica.
Neurology. 75: 2103-9. -
Coulpier, F. et al. (2010) CNS/PNS boundary transgression by central glia in the absence of Schwann cells or Krox20/Egr2 function.
J Neurosci. 30: 5958-67. -
Bramow, S. et al. (2010) Demyelination versus remyelination in progressive multiple sclerosis.
Brain.133: 2983-98. -
Kooij, G. et al. (2010) Adenosine triphosphate-binding cassette transporters mediate chemokine (C-C motif) ligand 2 secretion from reactive astrocytes: relevance to multiple sclerosis pathogenesis.
Brain. 134: 555-70. -
Kooi, E.J. et al. (2011) Cholinergic imbalance in the multiple sclerosis hippocampus.
Acta Neuropathol. 122: 313-22. -
Haider, L. et al. (2011) Oxidative damage in multiple sclerosis lesions.
Brain. 134: 1914-24. -
Baeten, K. et al. (2011) Tracking of myelin-reactive T cells in experimental autoimmune encephalomyelitis (EAE) animals using small particles of iron oxide and MRI.
NMR Biomed. 23: 601-9. -
Bagnato, F. et al. (2011) Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla.
Brain. 134: 3602-15. -
Hinson, S.R. et al. (2012) Molecular outcomes of neuromyelitis optica (NMO)-IgG binding to aquaporin-4 in astrocytes.
Proc Natl Acad Sci U S A. 109: 1245-50. -
Kooi, E.J. et al. (2012) Heterogeneity of cortical lesions in multiple sclerosis: clinical and pathologic implications.
Neurology. 79 (13): 1369-76. -
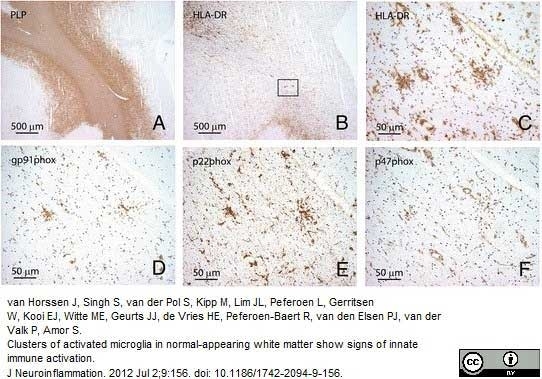
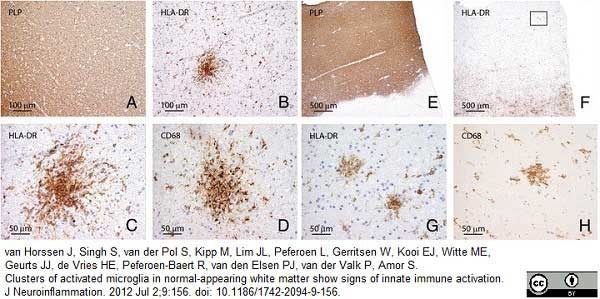
van Horssen, J. et al. (2012) Clusters of activated microglia in normal-appearing white matter show signs of innate immune activation.
J Neuroinflammation. 9: 156. -
Seewann, A. et al. (2012) Postmortem verification of MS cortical lesion detection with 3D DIR.
Neurology. 78: 302-8. -
Skripuletz, T. et al. (2013) Astrocytes regulate myelin clearance through recruitment of microglia during cuprizone-induced demyelination.
Brain. 136 (Pt 1): 147-67. -
Skripuletz, T. et al. (2015) Pivotal role of choline metabolites in remyelination.
Brain. 138 (Pt 2): 398-413. -
Fjær, S. et al. (2015) Magnetization transfer ratio does not correlate to myelin content in the brain in the MOG-EAE mouse model.
Neurochem Int. 83-84: 28-40. -
Klok, M.D. et al. (2015) Interferon-α and the calcifying microangiopathy in Aicardi-Goutières syndrome.
Ann Clin Transl Neurol. 2 (7): 774-9. -
Clarner, T. et al. (2015) CXCL10 triggers early microglial activation in the cuprizone model.
J Immunol. 194 (7): 3400-13. -
Alme, M.N. et al. (2015) Fingolimod does not enhance cerebellar remyelination in the cuprizone model.
J Neuroimmunol. 285: 180-6. -
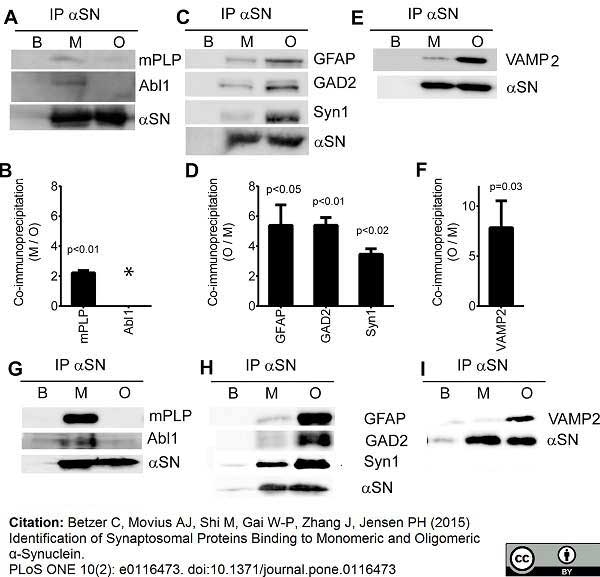
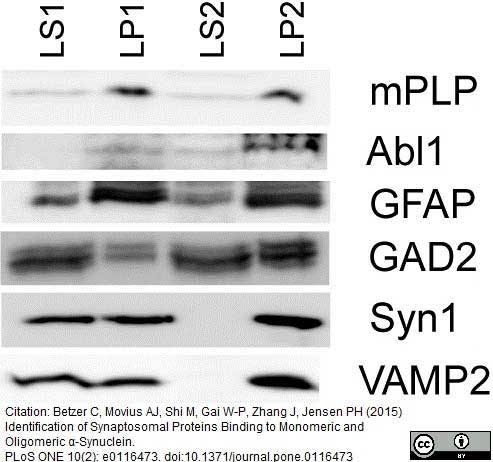
Betzer, C. et al. (2015) Identification of Synaptosomal Proteins Binding to Monomeric and Oligomeric α-Synuclein.
PLoS One. 10: e0116473. -
Jonkman, L.E. et al. (2016) Ultra-high field MTR and qR2* differentiates subpial cortical lesions from normal-appearing gray matter in multiple sclerosis.
Mult Scler. 22 (10): 1306-14. -
Kilsdonk, I.D. et al. (2016) Increased cortical grey matter lesion detection in multiple sclerosis with 7 T MRI: a post-mortem verification study.
Brain. 139 (Pt 5): 1472-81. -
Dooves, S. et al. (2016) Astrocytes are central in the pathomechanisms of vanishing white matter.
J Clin Invest. 126 (4): 1512-24. -
Russi, A.E. et al. (2016) Meningeal mast cell-T cell crosstalk regulates T cell encephalitogenicity.
J Autoimmun. 73: 100-10. -
Nakajima, M. et al. (2016) Auraptene induces oligodendrocyte lineage precursor cells in a cuprizone-induced animal model of demyelination.
Brain Res. 1639: 28-37. -
van Horssen, J. et al. (2016) Human endogenous retrovirus W in brain lesions: Rationale for targeted therapy in multiple sclerosis.
Mult Scler Relat Disord. 8: 11-8. -
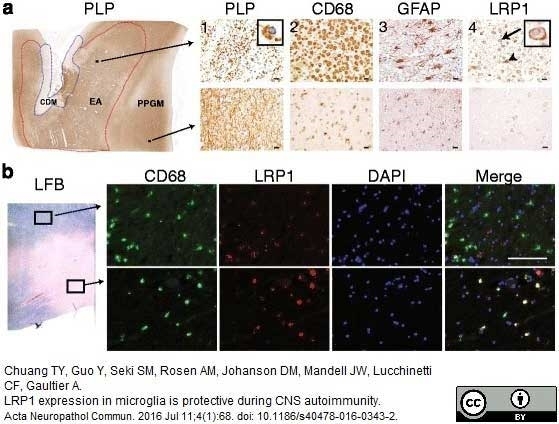
Chuang, T.Y. et al. (2016) LRP1 expression in microglia is protective during CNS autoimmunity.
Acta Neuropathol Commun. 4 (1): 68. -
Barateiro, A. et al. (2016) S100B as a Potential Biomarker and Therapeutic Target in Multiple Sclerosis.
Mol Neurobiol. 53 (6): 3976-91. -
Janssen, K. et al. (2016) Absence of CCL2 and CCL3 Ameliorates Central Nervous System Grey Matter But Not White Matter Demyelination in the Presence of an Intact Blood-Brain Barrier.
Mol Neurobiol. 53 (3): 1551-64. -
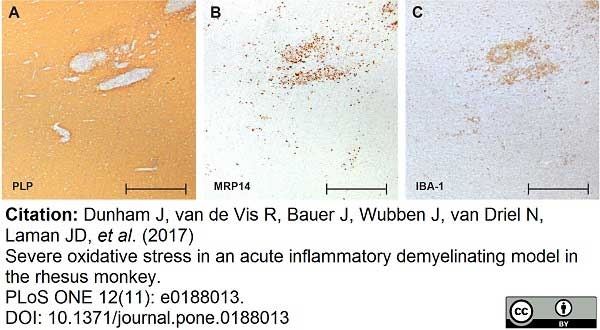
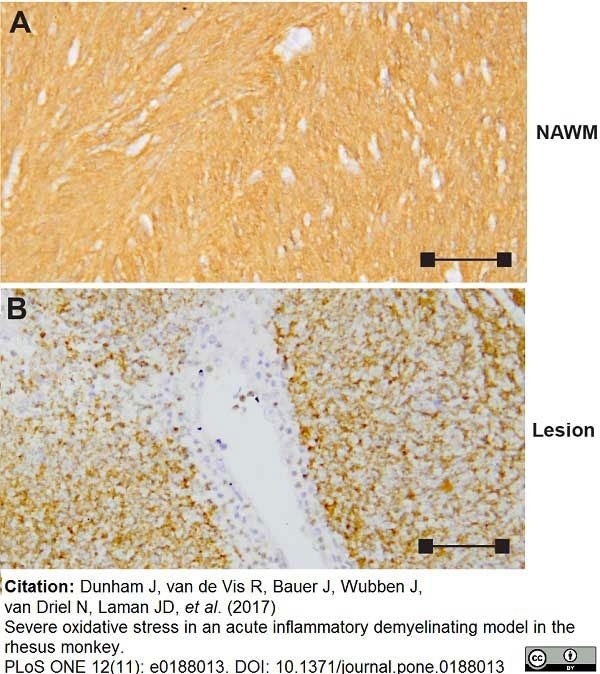
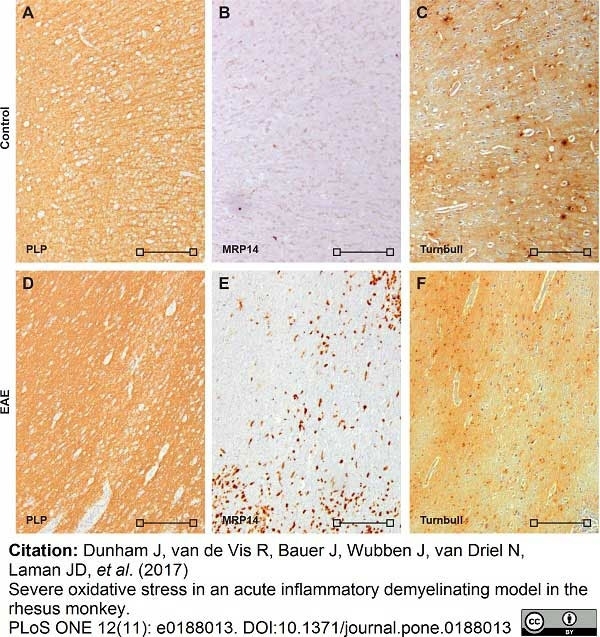
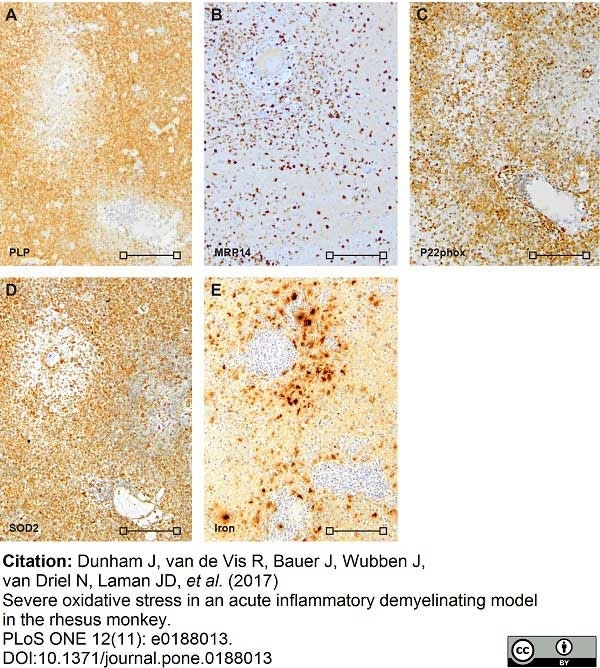
Dunham, J. et al. (2017) Severe oxidative stress in an acute inflammatory demyelinating model in the rhesus monkey.
PLoS One. 12 (11): e0188013. -
Bihler, K. et al. (2017) Formyl Peptide Receptor 1-Mediated Glial Cell Activation in a Mouse Model of Cuprizone-Induced Demyelination.
J Mol Neurosci. 62 (2): 232-43. -
Tobin, W.O. et al. (2017) Clinical-radiological-pathological spectrum of central nervous system-idiopathic inflammatory demyelinating disease in the elderly.
Mult Scler. 23 (9): 1204-13. -
Michailidou, I. et al. (2017) Complement C3 on microglial clusters in multiple sclerosis occur in chronic but not acute disease: Implication for disease pathogenesis.
Glia. 65 (2): 264-77. -
Maccarrone, G. et al. (2017) MALDI imaging mass spectrometry analysis-A new approach for protein mapping in multiple sclerosis brain lesions.
J Chromatogr B Analyt Technol Biomed Life Sci. 1047: 131-40. -
Cerina, M. et al. (2017) The quality of cortical network function recovery depends on localization and degree of axonal demyelination.
Brain Behav Immun. 59: 103-17. -
de Jong C. et al. (2018) Galectin-4, a Negative Regulator of Oligodendrocyte Differentiation, Is Persistently Present in Axons and Microglia/Macrophages in Multiple Sclerosis Lesions.
J Neuropathol Exp Neurol. 77 (11): 1024-38. -
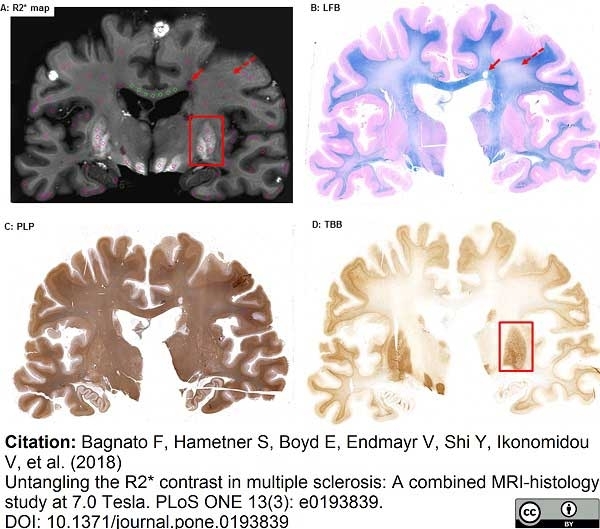
Bagnato, F. et al. (2018) Untangling the R2* contrast in multiple sclerosis: A combined MRI-histology study at 7.0 Tesla.
PLoS One. 13 (3): e0193839. -
Esser, S. et al. (2018) Toll-Like Receptor 2-Mediated Glial Cell Activation in a Mouse Model of Cuprizone-Induced Demyelination.
Mol Neurobiol. 55 (8): 6237-49. -
McKavanagh, R. et al. (2019) Relating diffusion tensor imaging measurements to microstructural quantities in the cerebral cortex in multiple sclerosis.
Hum Brain Mapp. 40 (15): 4417-31. -
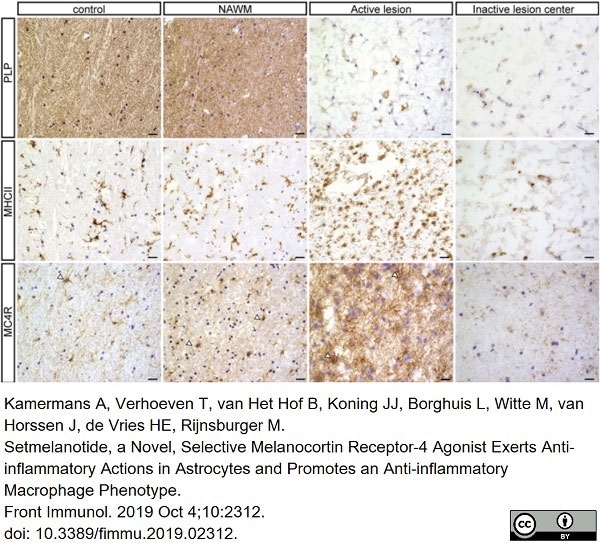
Kamermans, A. et al. (2019) Setmelanotide, a Novel, Selective Melanocortin Receptor-4 Agonist Exerts Anti-inflammatory Actions in Astrocytes and Promotes an Anti-inflammatory Macrophage Phenotype.
Front Immunol. 10: 2312. -
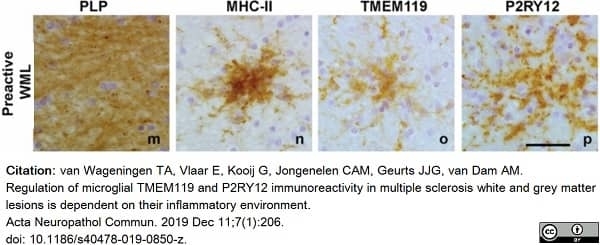
van Wageningen, T.A. et al. (2019) Regulation of microglial TMEM119 and P2RY12 immunoreactivity in multiple sclerosis white and grey matter lesions is dependent on their inflammatory environment.
Acta Neuropathol Commun. 7 (1): 206. -
Rohr, S.O. et al. (2020) Aquaporin-4 Expression during Toxic and Autoimmune Demyelination.
Cells. 9 (10): 2187. -
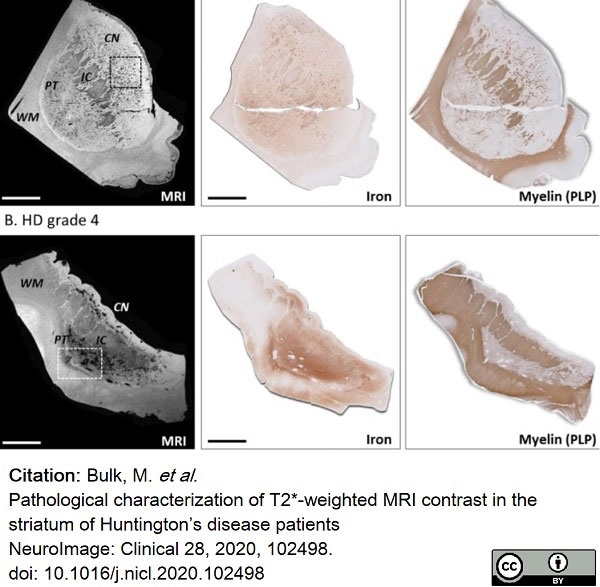
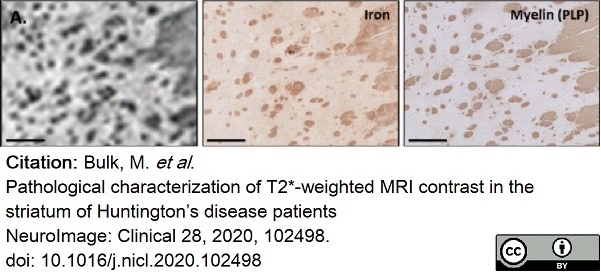
Bulk, M. et al. (2020) Pathological characterization of T2*-weighted MRI contrast in the striatum of Huntington’s disease patients
NeuroImage: Clinical. 28: 102498. -
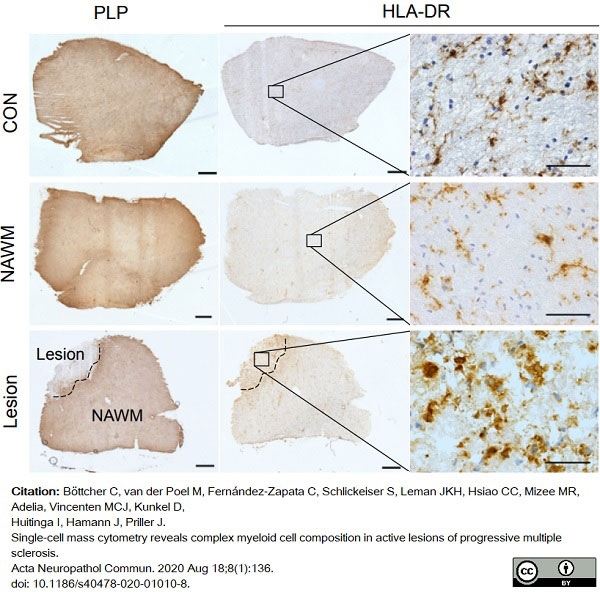
Böttcher, C. et al. (2020) Single-cell mass cytometry reveals complex myeloid cell composition in active lesions of progressive multiple sclerosis.
Acta Neuropathol Commun. 8 (1): 136. -
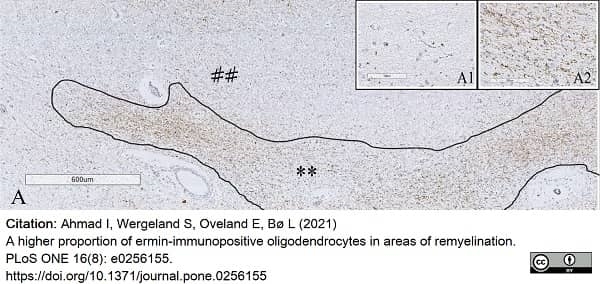
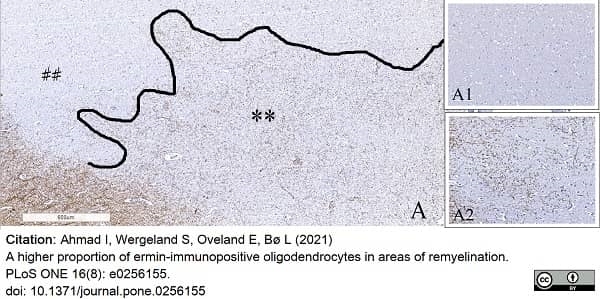
Ahmad, I. et al. (2021) A higher proportion of ermin-immunopositive oligodendrocytes in areas of remyelination.
PLoS One. 16 (8): e0256155. -
Baksmeier, C. et al. (2021) Modified recombinant human IgG1-Fc is superior to natural intravenous immunoglobulin at inhibiting immune-mediated demyelination.
Immunology. 164 (1): 90-105. -
Gudi, V. et al. (2021) Regenerative Effects of CDP-Choline: A Dose-Dependent Study in the Toxic Cuprizone Model of De- and Remyelination
Pharmaceuticals. 14 (11): 1156. -
Tham, M. et al. (2021) Iron Heterogeneity in Early Active Multiple Sclerosis Lesions.
Ann Neurol. 89 (3): 498-510. -
Helman, G. et al. (2021) Cerebral Microangiopathy in Leukoencephalopathy With Cerebral Calcifications and Cysts: A Pathological Description.
J Child Neurol. 36 (2): 133-40. -
Kolb, H. et al. (2021) 7T MRI Differentiates Remyelinated from Demyelinated Multiple Sclerosis Lesions.
Ann Neurol. 90 (4): 612-26. -
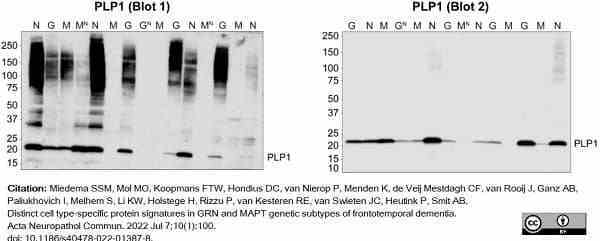
Miedema, A. et al. (2022) Brain macrophages acquire distinct transcriptomes in multiple sclerosis lesions and normal appearing white matter.
Acta Neuropathol Commun. 10 (1): 8. -
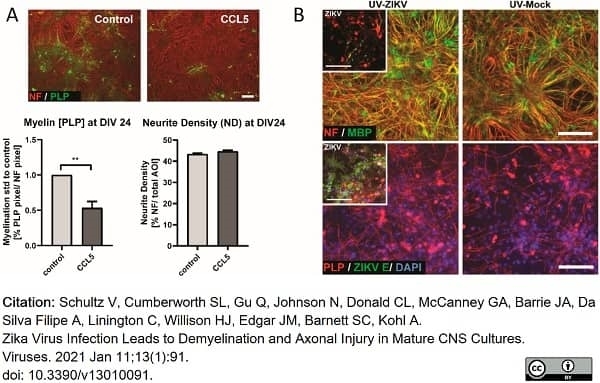
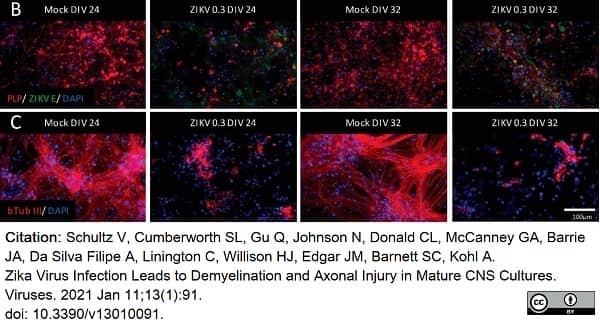
Schultz, V. et al. (2021) Zika Virus Infection Leads to Demyelination and Axonal Injury in Mature CNS Cultures.
Viruses. 13(1):91. -
Muñoz, U. et al. (2022) Main Role of Antibodies in Demyelination and Axonal Damage in Multiple Sclerosis.
Cell Mol Neurobiol. 42 (6): 1809-27. -
Miedema, S.S.M. et al. (2022) Distinct cell type-specific protein signatures in GRN and MAPT genetic subtypes of frontotemporal dementia.
Acta Neuropathol Commun. 10 (1): 100. -
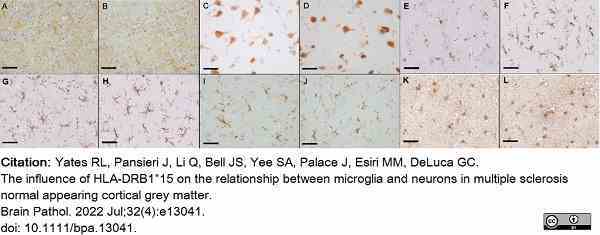
Yates, R.L. et al. (2022) The influence of HLA-DRB1*15 on the relationship between microglia and neurons in multiple sclerosis normal appearing cortical grey matter.
Brain Pathol. 32 (4): e13041. -
Hardy, T.A. et al. (2022) The clinical spectrum of haemorrhagic CNS inflammatory demyelinating lesions.
Mult Scler. 28 (11): 1710-8. -
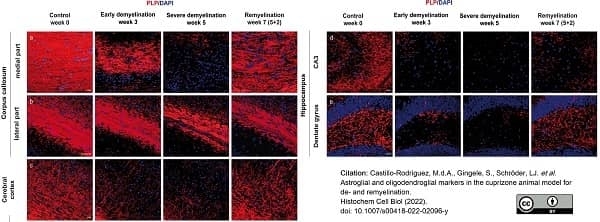
Castillo-Rodriguez, M.L.A. et al. (2022) Astroglial and oligodendroglial markers in the cuprizone animal model for de- and remyelination.
Histochem Cell Biol. 158 (1): 15-38. -
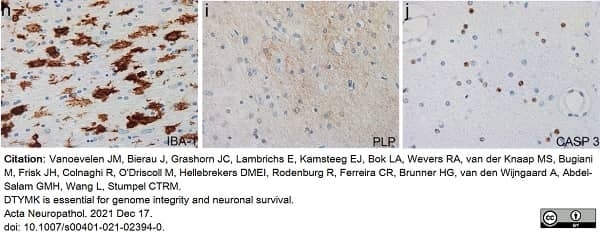
Vanoevelen, J.M. et al. (2022) DTYMK is essential for genome integrity and neuronal survival.
Acta Neuropathol. 143 (2): 245-62. -
Cooze, B.J. et al. (2022) The association between neurodegeneration and local complement activation in the thalamus to progressive multiple sclerosis outcome.
Brain Pathol. : e13054. -
Vanoevelen, J.M. et al. (2022) DTYMK is essential for genome integrity and neuronal survival.
Acta Neuropathol. 143 (2): 245-62. -
Miedema, A. et al. (2022) Brain macrophages acquire distinct transcriptomes in multiple sclerosis lesions and normal appearing white matter.
Acta Neuropathol Commun. 10 (1): 8. -
Guo, Y. et al. (2022) Spectrum of sublytic astrocytopathy in neuromyelitis optica.
Brain. 145 (4): 1379-90. -
van den Bosch, A. et al. (2022) Neurofilament Light Chain Levels in Multiple Sclerosis Correlate With Lesions Containing Foamy Macrophages and With Acute Axonal Damage.
Neurol Neuroimmunol Neuroinflamm. 9 (3): e1154. -
Valencia-Sanchez, C. et al. (2023) Cerebral Cortical Encephalitis in Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease.
Ann Neurol. 93 (2): 297-302. -
Wiggermann, V. et al. (2023) Quantitative magnetic resonance imaging reflects different levels of histologically determined myelin densities in multiple sclerosis, including remyelination in inactive multiple sclerosis lesions.
Brain Pathol. : e13150. -
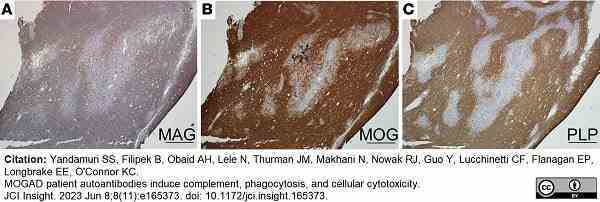
Yandamuri, S.S. et al. (2023) MOGAD patient autoantibodies induce complement, phagocytosis, and cellular cytotoxicity.
JCI Insight. 8 (11): e165373. -
Bekheet, E. & Sonbol, M. (2022) Evaluation of the Role of Growth Hormone Against Cuprizone Induced Multiple Sclerosis in the Cerebellar Cortex of Adult Female Albino Rat (Histological, Immunohistochemical and Radiological study)
Egyptian J Histol. 46 (3): 1322-40. -
Mailleux, J. et al. (2018) Active liver X receptor signaling in phagocytes in multiple sclerosis lesions.
Mult Scler. 24 (3): 279-89. -
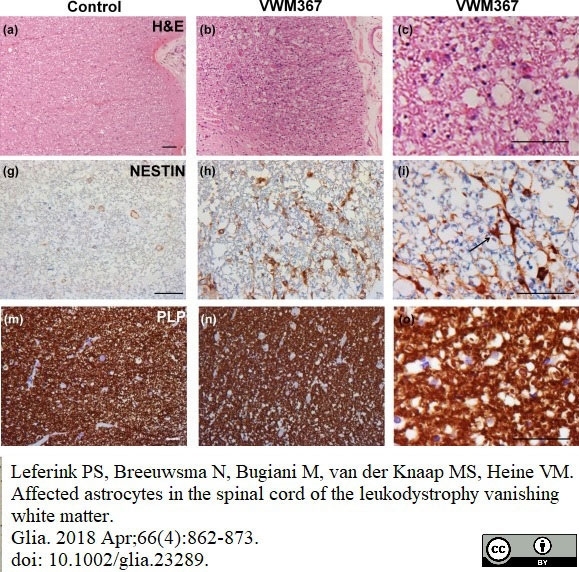
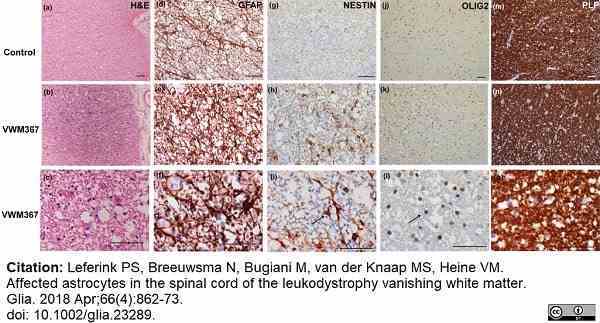
Leferink, P.S. et al. (2018) Affected astrocytes in the spinal cord of the leukodystrophy vanishing white matter.
Glia. 66 (4): 862-73. -
Klok, M.D. et al. (2018) Axonal abnormalities in vanishing white matter.
Ann Clin Transl Neurol. 5 (4): 429-44. -
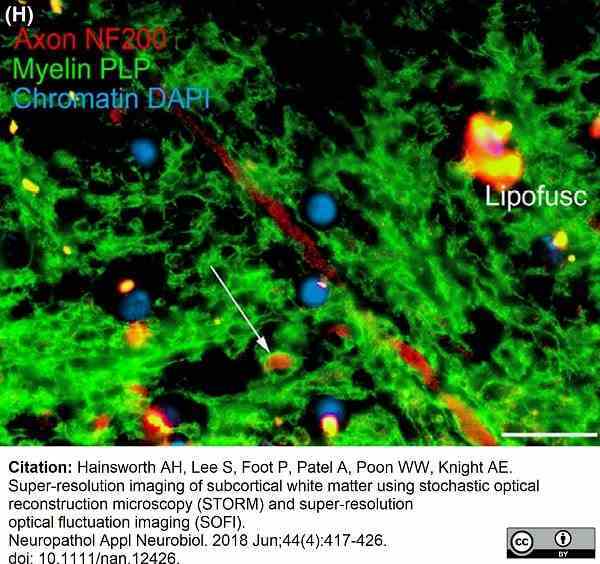
Hainsworth, A.H. et al. (2018) Super-resolution imaging of subcortical white matter using stochastic optical reconstruction microscopy (STORM) and super-resolution optical fluctuation imaging (SOFI).
Neuropathol Appl Neurobiol. 44 (4): 417-26.
- Synonyms
- DM20
- PLP
- RRID
- AB_2237198
- UniProt
- P04116
- Entrez Gene
- PLP1
- GO Terms
- GO:0016021 integral to membrane
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Bovine ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up