CD8 antibody | CC63













Mouse anti Bovine CD8
- Product Type
- Monoclonal Antibody
- Clone
- CC63
- Isotype
- IgG2a
- Specificity
- CD8
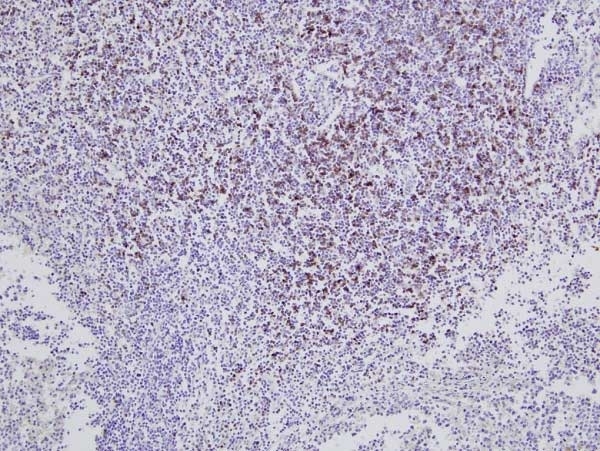
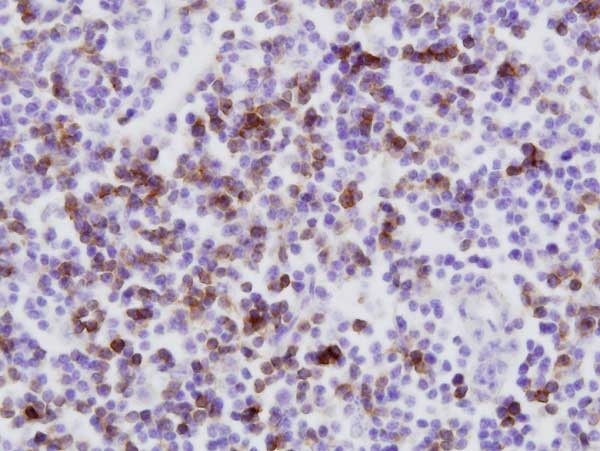
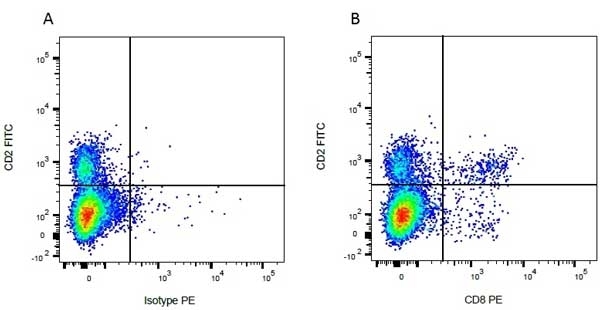
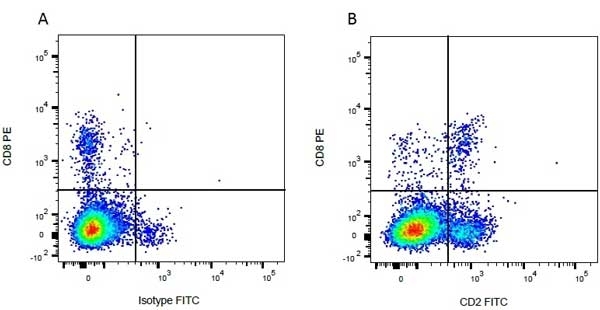
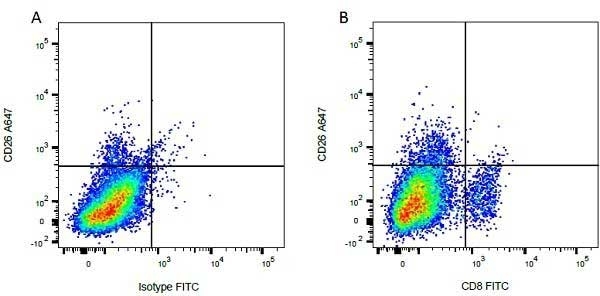
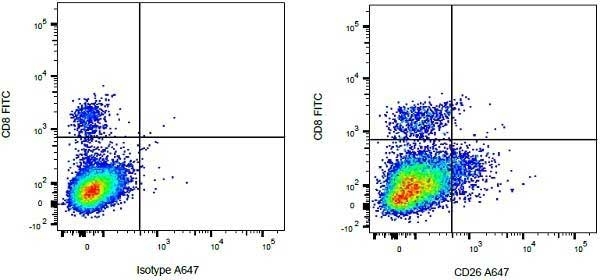
| Mouse anti Bovine CD8 antibody, clone CC63 reacts with the bovine CD8 antigen expressed by a subset of T lymphocytes. The antibody precipitates molecules of ~34 kDa and ~38 kDa under reducing conditions. Clone CC63 has been reported as being suitable for use on formalin dichromate (FD5) fixed paraffin embedded tissue with amplification and antigen retrieval techniques (Gutierrez et al. 1999). |
- Target Species
- Bovine
- Species Cross-Reactivity
-
Target Species Cross Reactivity Sheep Goat - N.B. Antibody reactivity and working conditions may vary between species.
- Product Form
- Purified IgG - liquid
- Preparation
- Purified IgG prepared by affinity chromatography on Protein G from tissue culture supernatant
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
- 0.09% Sodium Azide (NaN3)
- Carrier Free
- Yes
- Approx. Protein Concentrations
- IgG concentration 1 mg/ml
- Fusion Partners
- Spleen cells from an immunized mouse were fused with cells of the mouse NS1 myeloma cell line.
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
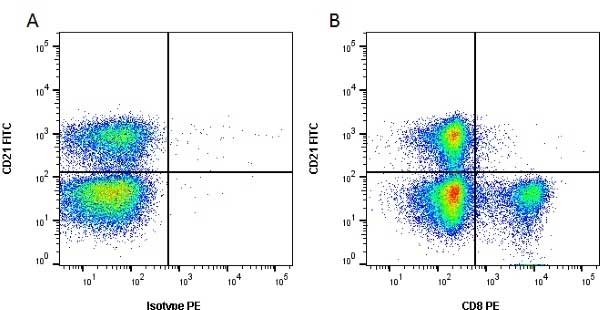
| Flow Cytometry | 1/50 | ||
| Immunofluorescence | |||
| Immunohistology - Frozen | |||
| Immunohistology - Paraffin | |||
| Immunoprecipitation |
- Flow Cytometry
- Use 10ul of the suggested working dilution to label 106 cells in 100ul.
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Mouse IgG2a Negative Control | MCA929 | F | 100 Tests |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Mouse IgG2a Negative Control | ||||||
References for CD8 antibody
-
MacHugh, N.D. & Sopp P (1991) Individual antigens of cattle. Bovine CD8 (BoCD8).
Vet Immunol Immunopathol. 27 (1-3): 65-9. -
Gutierrez, M. et al. (1999) The detection of CD2+, CD4+, CD8+, and WC1+ T lymphocytes, B cells and macrophages in fixed and paraffin embedded bovine tissue using a range of antigen recovery and signal amplification techniques.
Vet Immunol Immunopathol. 71 (3-4): 321-34. -
Winkler, M.T. et al. (1999) Bovine herpesvirus 1 can infect CD4(+) T lymphocytes and induce programmed cell death during acute infection of cattle.
J Virol. 73 (10): 8657-68. -
Winkler, M.T. et al. (2000) Persistence and reactivation of bovine herpesvirus 1 in the tonsils of latently infected calves.
J Virol. 74 (11): 5337-46. -
Twizere, J.C. et al. (2000) Discordance between bovine leukemia virus tax immortalization in vitro and oncogenicity in vivo.
J Virol. 74 (21): 9895-902. -
Harris, J. et al. (2002) Expression of caveolin by bovine lymphocytes and antigen-presenting cells.
Immunology. 105: 190-5. -
Toman, M. et al. (2003) Immunological characteristics of cale with Mycobacterium avium subsp. paratuberculosis infection
Vet. Med. – Czech, 48, 2003: 147–54. -
Vordermeier, H.M. et al. (2004) Cellular immune responses induced in cattle by heterologous prime-boost vaccination using recombinant viruses and bacille Calmette-Guérin.
Immunology. 112: 461-70.
View The Latest Product References
-
Vitale, F. et al. (2006) ESAT-6 peptide recognition by bovine CD8+ lymphocytes of naturally infected cows in herds from southern Italy.
Clin Vaccine Immunol. 13: 530-3. -
Fulton, B.E. Jr. et al. (2006) Dissemination of bovine leukemia virus-infected cells from a newly infected sheep lymph node.
J Virol. 80: 7873-84. -
Liebana, E. et al. (2007) Distribution and activation of T-lymphocyte subsets in tuberculous bovine lymph-node granulomas.
Vet Pathol. 44: 366-72. -
Foulon, E. & Foucras, G. (2008) Two populations of ovine bone marrow-derived dendritic cells can be generated with recombinant GM-CSF and separated on CD11b expression.
J Immunol Methods. 339 (1): 1-10. -
Sidders, B. et al. (2008) Screening of highly expressed mycobacterial genes identifies Rv3615c as a useful differential diagnostic antigen for the Mycobacterium tuberculosis complex.
Infect Immun. 76: 3932-9. -
Lynch, E.M. et al. (2010) Effect of abrupt weaning at housing on leukocyte distribution, functional activity of neutrophils, and acute phase protein response of beef calves.
BMC Vet Res. 6: 39. -
Coad, M. et al. (2010) Repeat tuberculin skin testing leads to desensitisation in naturally infected tuberculous cattle which is associated with elevated interleukin-10 and decreased interleukin-1 beta responses.
Vet Res. 41: 14. -
Constantinoiu, C.C. et al. (2010) Local immune response against larvae of Rhipicephalus (Boophilus) microplus in Bos taurus indicus and Bos taurus taurus cattle.
Int J Parasitol. 40: 865-75. -
La Manna, M.P. et al. (2011) Expansion of intracellular IFN-γ positive lymphocytes during Mycoplasma agalactiae infection in sheep.
Res Vet Sci. 91 (3): e64-7. -
Sanchez, J. et al. (2011) Microscopical and immunological features of tuberculoid granulomata and cavitary pulmonary tuberculosis in naturally infected goats.
J Comp Pathol. 145 (2-3): 107-17. -
Lacroux, C. et al. (2012) Prionemia and leukocyte-platelet-associated infectivity in sheep transmissible spongiform encephalopathy models.
J Virol. 86 (4): 2056-66. -
Brodzki, P. et al. (2014) Phenotyping of leukocytes and granulocyte and monocyte phagocytic activity in the peripheral blood and uterus of cows with endometritis.
Theriogenology. 82 (3): 403-10. -
Silva, A.P. et al. (2015) Encapsulated Brucella ovis Lacking a Putative ATP-Binding Cassette Transporter (&Detla;abcBA) Protects against Wild Type Brucella ovis in Rams.
PLoS One. 10 (8): e0136865. -
Leite FL et al. (2015) ZAP-70, CTLA-4 and proximal T cell receptor signaling in cows infected with Mycobacterium avium subsp. paratuberculosis.
Vet Immunol Immunopathol. 167 (1-2): 15-21. -
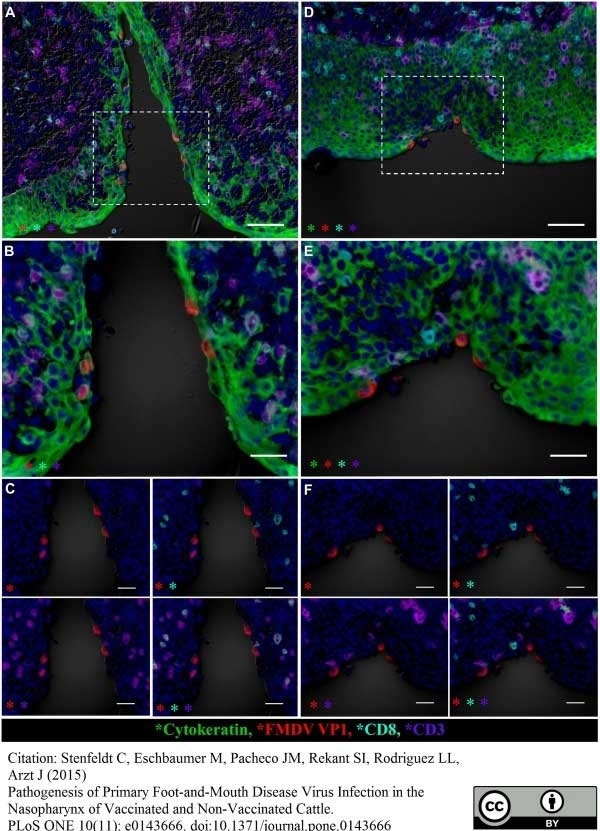
Stenfeldt, C. et al. (2015) Pathogenesis of Primary Foot-and-Mouth Disease Virus Infection in the Nasopharynx of Vaccinated and Non-Vaccinated Cattle.
PLoS One. 10 (11): e0143666. -
Romero-Palomo, F. et al. (2017) Immunopathologic Changes in the Thymus of Calves Pre-infected with BVDV and Challenged with BHV-1.
Transbound Emerg Dis. 64 (2): 574-84. -
Schmidt, N. et al. (2018) Decreased STEC shedding by cattle following passive and active vaccination based on recombinant Escherichia coli Shiga toxoids.
Vet Res. 49 (1): 28. -
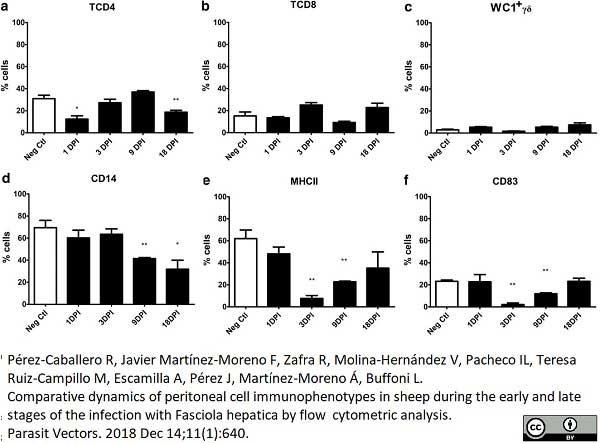
Pérez-caballero, R. et al. (2018) Comparative dynamics of peritoneal cell immunophenotypes in sheep during the early and late stages of the infection with Fasciola hepatica by flow cytometric analysis.
Parasit Vectors. 11 (1): 640. -
Benedictus, L. et al. (2019) Immunization of young heifers with staphylococcal immune evasion proteins before natural exposure to Staphylococcus aureus induces a humoral immune response in serum and milk.
BMC Vet Res. 15 (1): 15. -
Nakajima, N. et al. (2019) Effects of direct exposure to cold weather under grazing in winter on the physiological, immunological, and behavioral conditions of Japanese Black beef cattle in central Japan.
Anim Sci J. 90 (8): 1033-41. -
de Araújo, F.F. et al. (2019) Distinct immune response profile during Rhipicephalus (Boophilus) microplus. infestations of guzerat dairy herd according to the maternal lineage ancestry (mitochondrial DNA).
Vet Parasitol. 273: 36-44. -
Kolar, Q.K. et al. (2020) Anatomical distribution of respiratory tract leukocyte cell subsets in neonatal calves.
Vet Immunol Immunopathol. 227: 110090. -
Risalde, M.A. et al. (2020) BVDV permissiveness and lack of expression of co-stimulatory molecules on PBMCs from calves pre-infected with BVDV.
Comp Immunol Microbiol Infect Dis. 68: 101388. -
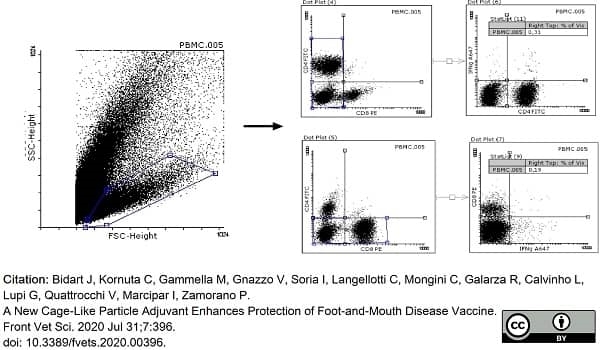
Bidart, J. et al. (2020) A New Cage-Like Particle Adjuvant Enhances Protection of Foot-and-Mouth Disease Vaccine.
Front Vet Sci. 7: 396. -
Brodzki, P. et al. (2020) Selected leukocyte subpopulations in peripheral blood and uterine washings in cows before and after intrauterine administration of cefapirin and methisoprinol.
Anim Sci J. 91 (1): e13306. -
Bloomer, S.A. et al. (2020) Aging results in accumulation of M1 and M2 hepatic macrophages and a differential response to gadolinium chloride.
Histochem Cell Biol. 153 (1): 37-48. -
Gondaira, S. et al. (2020) Immunosuppression in Cows following Intramammary Infusion of Mycoplasma bovis.
Infect Immun. 88 (3) :e00521-19. -
Damani-Yokota, P. et al. (2021) Transcriptional programming and gene regulation in WC1+ γδ T cell subpopulations.
Mol Immunol. 142: 50-62. -
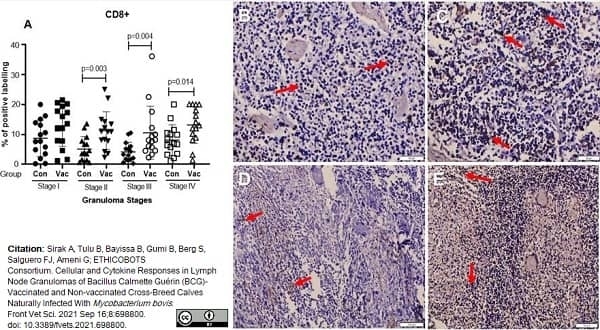
Sirak, A. et al. (2021) Cellular and Cytokine Responses in Lymph Node Granulomas of Bacillus Calmette Guérin (BCG)-Vaccinated and Non-vaccinated Cross-Breed Calves Naturally Infected With Mycobacterium bovis.
Front Vet Sci. 8: 698800. -
Colombatti, M.O. et al. (2021) Evaluation of a virulent strain of Mycobacterium avium subsp. paratuberculosis used as a heat-killed vaccine.
Vaccine. 39 (51): 7401-12. -
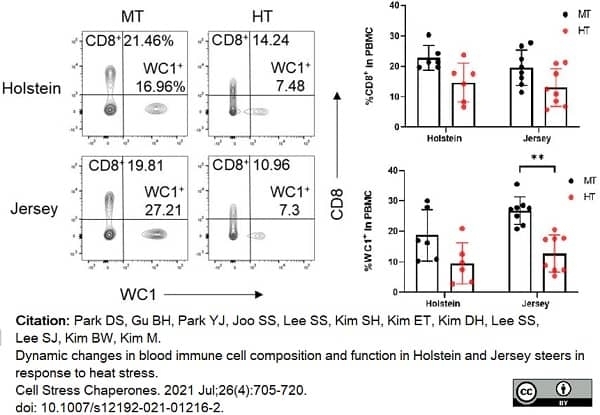
Park, D.S. et al. (2021) Dynamic changes in blood immune cell composition and function in Holstein and Jersey steers in response to heat stress.
Cell Stress Chaperones. 26 (4): 705-20. -
Nashiruddullah, N. et al. (2021) Dermal Response to Experimental Orfvirus (ORFV) Infection in Goats, Mice and Rabbit
Indian J Anim Res. 56 (8): B-4266 1003-9. -
Kato-Mori, Y. et al. (2021) Characterization of a variant CD4 molecule in Japanese Black cattle.
Vet Immunol Immunopathol. 232: 110167. -
Casaro, S. et al. (2022) Flow cytometry panels for immunophenotyping dairy cattle peripheral blood leukocytes
VetImmunol Immunopathol. 248: 110417. -
Elsayed, M.S.A.E. et al. (2022) Real-time PCR using atpE, conventional PCR targeting different regions of difference, and flow cytometry for confirmation of Mycobacterium bovis. in buffaloes and cattle from the Delta area of Egypt.
BMC Microbiol. 22 (1): 154. -
Korbonits, L. et al. (2022) Mycobacterium avium subsp. paratuberculosis Infected Cows Reveal Divergent Immune Response in Bovine Peripheral Blood Derived Lymphocyte Proteome.
Metabolites. 12 (10): 924. -
Tucker, N. et al. (2023) Bovine blood and milk T-cell subsets in distinct states of activation and differentiation during subclinical Staphylococcus aureus mastitis.
J Reprod Immunol. 156: 103826. -
Özbek, M. & Bayraktaroğlu, A.G. (2019) Developmental study on the ileal Peyer's patches of sheep, and cytokeratin-18 as a possible marker for M cells in follicle associated epithelium.
Acta Histochem. 121 (3): 311-22. -
Benedictus, L. et al. (2019) Immunization of young heifers with staphylococcal immune evasion proteins before natural exposure to Staphylococcus aureus induces a humoral immune response in serum and milk.
BMC Vet Res. 15 (1): 15. -
Yang, L. et al. (2018) Association of the expression of Th cytokines with peripheral CD4 and CD8 lymphocyte subsets after vaccination with FMD vaccine in Holstein young sires.
Res Vet Sci. 119: 79-84. -
Andrés, S. et al. (2024) Essential oil supplementation in milk replacers: short- and long-term impacts on feed efficiency, the faecal microbiota and the plasma metabolome in dairy calves.
J Dev Orig Health Dis. : 1-11.
- UniProt
- P31783
- Entrez Gene
- CD8A
- GO Terms
- GO:0016021 integral to membrane
MCA837GA
If you cannot find the batch/lot you are looking for please contact our technical support team for assistance.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Bovine ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up