What Is alamarBlue?
Sizes Available
alamarBlue can be used for applications such as functional assay, immunofluorescence, and ELISA.
This reagent is offered in two sizes:
25 ml = Enough for 2,500 wells
/96-well plate
100 ml = Enough for 10,000 wells
/96-well plate
Note: Calculations assume 100 µl final volume per well (96-well plate).
Overview
alamarBlue is a cell viability assay reagent which contains the cell permeable, non-toxic, and weakly fluorescent blue indicator dye called resazurin. This is a trusted and established reagent which has been available since 1993. alamarBlue is a useful non-toxic alternative to the commonly used MTT cell viability assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide) (O’Brien et al. 2000).
alamarBlue quantitatively measures proliferation in human, animal, bacterial, fungal, and mycobacterial cells. It is useful for cytokine bioassays, cell viability assays, and in vitro cytotoxicity determinations as well as cell growth monitoring.
How Does alamarBlue Work?
Resazurin is used as an oxidation-reduction (REDOX) indicator that undergoes colorimetric change in response to cellular metabolic reduction. The reduced form, resorufin, is pink and highly fluorescent, and the intensity of fluorescence produced is proportional to the number of living cells respiring. alamarBlue is a direct indicator of cell health, it detects the level of oxidation during respiration, quantitatively measuring cell viability and cytotoxicity.
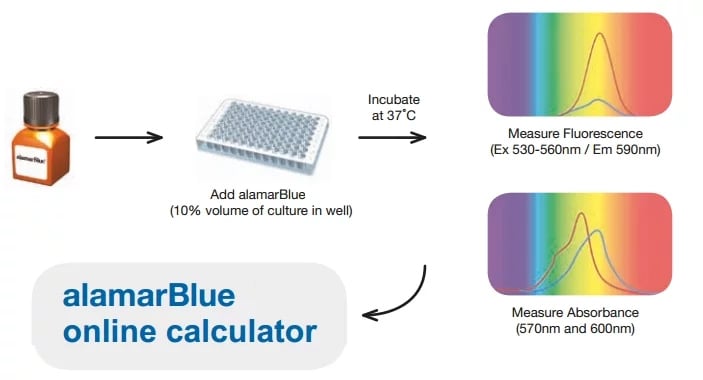
Our how to use alamarBlue page will provide you with useful methods and calculations for you to plan your experiments.
How alamarBlue Acts as a Redox Indicator
The midpoint potential (Eo’) for any half reaction can be defined as the voltage at which equilibrium is obtained between the concentration of oxidized and reduced products. This midpoint potential gives a measure of the strength of an electron donor. For example, in an equation, a component with a more negative midpoint potential is a stronger electron donor, making it a weaker electron acceptor. Example midpoint potentials are given in Table 1. If any two half equations are combined, the midpoint potentials can be used to determine in which direction the electrons will flow.
Table 1. Oxidation reduction potentials in the electron transport system and alamarBlue.
| Half-reaction | Eo' (mV) pH7.0 25oC |
|||
|---|---|---|---|---|
| NAD+ 2H+ + 2e- | <----> | NADH + H+ | -320 | |
| NADP+ 2H+ + 2e- | <----> | NADPH + H+ | -320 | |
| FAD+ 2H+ + 2e- | <----> | FADH2 | -220 | |
| FMN+ 2H+ + 2e- | <----> | FMNH2 | -210 | |
| MTTOX1 + 2H+ + 2e- | <----> | MTTRED | -110 | |
| cytochromesOX1 +1 e- | <----> | cytochromesRED | +80 to +290 | |
| alamarBlueOX + 2H+ + 2e- | <----> | alamarBlueRED | 380 | |
| O2 + 4H+ + 4e- | <----> | 2H2O | 820 | |
Note: The midpoint redox potential (Eo’) values were determined at pH 7.0, at 25°C.
alamarBlue is an excellent redox indicator for cell proliferation and viability studies because it does not interfere in any way with the reactions of the electron transport chain. With alamarBlue, the cells remain fully functional, viable, and healthy, unaffected by the presence of the indicator, which is a property unique to alamarBlue.
Here is why:
alamarBlue cell viability indicator is ideally set up to detect oxidation by the whole of the electron transport chain. As can be seen from Table 1, the midpoint potential of alamarBlue is greater than that of any of the cytochromes, allowing it to detect oxidation by all the components of the electron transport chain. alamarBlue can be reduced by FMNH2, FADH2, NADH, NADPH, and cytochromes, since their midpoint potentials are lower than that for alamarBlue.
How MTT Interrupts the Electron Transport Chain
An alternative redox indicator is tetrazolium salt (MTT), which has a midpoint potential of -110 mV (see Table 1). This enables MTT to be reduced by the electron donors FMNH2, FADH2, NADH, and NADPH, however, since the midpoint potential of MTT is intermediate between that of the electron donors and cytochromes, MTT cannot be reduced by the cytochromes.
The chain is broken when MTT is reduced because the electrons released by the donors will not be passed to the cytochromes as would normally happen in the electron transport chain. This stops the production from the electron transport chain which inhibits respiration, leading to cell death.
Reference
- O’Brien J et al. (2003). Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. The FEBS Journal, 267, 5421-5426




