alamarBlue FAQs

- On This Page
- Frequently asked questions
- References
Frequently Asked Questions
- Which cells can I use for alamarBlue tests?
- Will phenol red affect the measurements for alamarBlue?
- Which media can be used with alamarBlue?
- Will alamarBlue be toxic to my cells?
- Will microbial contaminants or carrier protein interfere with alamarBlue measurements?
- Why is it necessary to optimize the cell density used for studies with alamarBlue?
- There seems to be a decrease in the reduction of alamarBlue if I use a long incubation time?
- Can I use alamarBlue to repeatedly measure cell proliferation at intervals of days or weeks?
- Is it possible to use different filters than 570 nm and 600 nm for measuring cell growth by absorbance?
- Is it better to measure cell cytotoxicity using absorbance or fluorescence?
- Can I store my plates for measurement at a later date?
- Will temperature affect the fluorescence readings?
- Can alamarBlue be used for assays to measure lymphocyte proliferation?
- How can I determine the absorbance or fluorescence for the fully reduced form of alamarBlue?
- What are example absorbance and fluorescence values for the oxidized and reduced forms of alamarBlue?
Protocols for alamarBlue
1. Which cells can I use for alamarBlue tests?
alamarBlue has been successfully used with a wide range of cells including cultured mammalian cells, bacteria, yeast, fungi, and protozoa. To date, it has been suitable for use with most cell types tested. Whole blood and leukocytes are not recommended as the results are difficult to read and leukocytes metabolize the alamarBlue.
Back to FAQs list2. Will phenol red affect the measurements for alamarBlue?
There is no interference from the presence of phenol red in the growth medium. The presence of phenol red merely shifts the values approximately 0.03 units higher (see Table 1).
Table 1. Effect of phenol red on absorbance values at 570 nm.
| Media | Media Absorbance Value |
Absorbance Value for alamarBlue at Various Levels of Reduction | |||||
|---|---|---|---|---|---|---|---|
|
|
0% | 10% | 30% | 60% | 90% | 100% | |
|
RPMI 1640 without
phenol red
|
0.032 | 0.47 | 0.52 | 0.61 | 0.73 | 0.85 | 0.88 |
|
RPMI 1640 with
phenol red
|
0.061 | 0.53 | 0.54 | 0.64 | 0.76 | 0.88 | 0.91 |
Note: Absorbance value for various levels of reduction of alamarBlue in RPMI 1640 pH 7.0, with MOPS (both with and without phenol red) when using Dynatech flat bottom plates and 100 µl per well.
Back to FAQs list3. Which media can be used with alamarBlue?
Most commonly used media are suitable for use with alamarBlue assays. Since the media is used in the blank, and also any other positive or negative control plates, any effects of the media on absorbance or fluorescence should be minimal. Refer to Frequently Asked Question 15 for example data for oxidized and reduced forms of alamarBlue using a range of different media.
Back to FAQs list4. Will alamarBlue be toxic to my cells?
alamarBlue has undergone extensive testing, and is non toxic to all cell types tested. It has also been shown to have neither a positive or negative effect on cell growth.
Unlike other cell proliferation redox indicators, alamarBlue does not interfere with the electron transport chain, and does not affect cell respiration or function. Please visit our what is alamarBlue? page for further information about the redox determinants of alamarBlue.
Back to FAQs list5. Will microbial contaminants or carrier protein interfere with alamarBlue measurements?
alamarBlue will be reduced by microbial contaminants. Therefore, results from contaminated cultures tested by this method will not be accurate.
In-house studies indicated samples with protein concentrations equivalent to 10% fetal bovine serum (FBS) did not interfere with the assay. However, Page et al. (1993) reported that serum may cause some quenching of fluorescence and recommended using the same serum concentration in controls to take this into account. Goegan et al. (1995) tested the effects of varying concentrations of FBS, bovine serum albumin (BSA), and polyvinylpyrrolidone on the alamarBlue assay. Results showed that increasing concentrations of FBS and BSA did affect the assay. A method and calculation was developed to analyze the test matrix for effects due to these two compounds; allowing the correction of such effects. This calculation can then be applied to determine the consequence of any additive test media matrix, including the test chemicals themselves Goegan et al. (1995).
Back to FAQs list6. Why is it necessary to optimize the cell density used for studies with alamarBlue?
alamarBlue measures cell proliferation most accurately when the cells are in the exponential growth phase. If the cell density is too high, cell proliferation will decrease, giving less reduction of the alamarBlue than would have been expected. At low cell density, the slower growth rate could result in insignificant alamarBlue reduction. A cell density of 1 x 104 cells/ml is generally recommended, which often corresponds to cells being in the exponential phase. However, since cells vary in their proliferation rate, it is impossible to recommend a cell density which is suitable for all experiments. Instead we recommend that customers perform a control experiment to determine the optimum cell density for their studies. It is equally important to optimize the incubation time for alamarBlue. Please refer to alamarBlue example experiments for the general method to determine the optimum cell density and incubation time.
Fig. 1. A standard bacterial growth curve.
Back to FAQs list7. There seems to be a decrease in the reduction of alamarBlue if I use a long incubation time?
alamarBlue contains proprietary buffering agents, which maintain the equilibrium between the oxidized and reduced forms of the indicator. If alamarBlue is used for extended incubation periods, there is a possibility that the buffering agents will reach their maximum buffering efficiency. As this point, the equilibrium between the oxidized and reduced forms of alamarBlue is disrupted, and a colorless form of the dye may be obtained. This could result in an apparent bleaching of the solution, and a decrease in the amount of the reduced (red) form of alamarBlue.
If a decrease in reduction of the solution is observed at a certain point, or if any apparent bleaching is observed, we recommend that the incubation time is shortened. Please refer to alamarBlue example experiments for details of control experiments to optimize incubation time for alamarBlue.
Alternatively, if the length of the experiment is longer than the optimal alamarBlue incubation time, we suggest that an endpoint test is used. This type of experiment is particularly useful for cell proliferation studies over days, weeks, and months. For more information, please refer to Frequently Asked Question 8 below.
Back to FAQs list8. Can I use alamarBlue to repeatedly measure cell proliferation at intervals of days or weeks?
alamarBlue can be used for long term cell proliferation studies where measurements are taken repeatedly. For these types of study we would recommend that aliquots of the cell medium / suspension are taken at each time point for incubation with alamarBlue prior to an endpoint test. Please refer to Breinholt et al. (1998) for an example of this type of experiment.
Back to FAQs list9. Is it possible to use different filters than 570 nm and 600 nm for measuring cell growth by absorbance?
In the general method section of the datasheet, the extinction coefficients are given for filters at 540 nm, 570 nm, 600 nm, and 630 nm.
Rather than deriving molecular extinction coefficients for other filters, which may be subject to error, a simple and accurate method has been devised, to calculate the amount of reduced alamarBlue present, for any filter combination. (Personal communication of Dr. Geier and in independently derived calculations by Goegan et. al. (1995). For more information, please refer to example calculations for using different filters in the the Protocols for alamarBlue section.
Back to FAQs list10. Is it better to measure cell cytotoxicity using absorbance or fluorescence?
alamarBlue reduction is regularly measured using absorbance, which gives good levels of accuracy for most experiments, and is particularly easy to use.
It is generally the case, however, that more sensitive readings can be obtained when fluorescence is used, especially when attempting to measure very small changes in reduction. This is principally because there is little interference from non reduced alamarBlue on the spectra of reduced alamarBlue when measured by fluorescence (Figure 2a). When measuring absorption there can be considerable overlap of the oxidized and reduced forms of alamarBlue (Figure 2b).
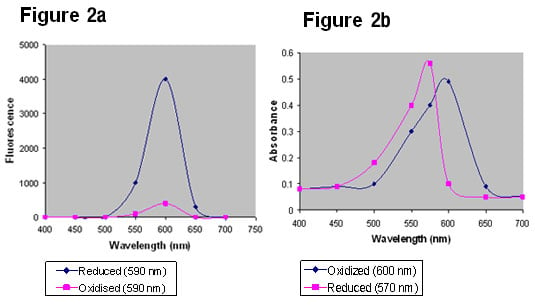
Fig. 2a & 2b. alamarBlue fluorescence emission spectra and absorbance readings.
Back to FAQs list11. Can I store my plates for measurement at a later date?
If it is not possible to read plates on the day an experiment is performed, plates may be refrigerated and read within 1-3 days. Plates should be wrapped in foil or plastic wrap to prevent evaporation and stored in the dark.
Please refer to Appendix 4 in the technical datasheet for example absorbance data for alamarBlue plates where readings were taken up to 3 days after the end of incubation.
N.B. If plates are refrigerated and fluorescence measurements are being used, keep in mind that fluorescence measurements are influenced by temperature. If measurements are normally taken at 37°C, then plates should be warmed to that temperature before reading.
Back to FAQs list12. Will temperature affect the fluorescence readings?
Fluorescence measurements are influenced by temperature. It is recommended that the temperature used for fluorescence readings remains constant, and that if plates are refrigerated they are warmed to that temperature before reading.
Table 2. Effect of temperature on fluorescence.
| Temperature | Fluorescence |
|---|---|
| 37oC | (171.1) |
| 5,216 | |
| 22oC | (157.6) |
| 5,887 | |
| 4oC | (192.1) |
| 6,881 |
Standard deviations are in parentheses (calculated for n=8). RPMI 1640 with MOPS pH7.0, no phenol red at 100 µl per well, using Dynatech flat bottom plate.
Back to FAQs list13. Can alamarBlue be used for assays to measure lymphocyte proliferation?
Recent studies have shown that alamarBlue may be used successfully to measure lymphocyte proliferation (Simms et al. 2000, Qureshi et al. 2001), although one study by De Fries et Mitsuhashi. (1995) has suggested that alamarBlue may not give such as sensitive reading as the traditional 3H-thymidine incorporation assays.
Back to FAQs list14. How can I determine the absorbance or fluorescence for the fully reduced form of alamarBlue?
The reduced form of alamarBlue is very unstable in water. For this reason, it is difficult to recommend a standard test for the reduced form. However, the reduced form is very stable in media. To determine the absorbance / fluorescence to be expected from the reduced form for a particular experiment, it is suggested that media containing 10% alamarBlue be made up in an autoclavable container. Reduce this preparation by autoclaving for 15 minutes. Remove from the autoclave and allow to cool to room temperature. Swirl the solution several times before taking the appropriate measurements.
Back to FAQs list15. What are example absorbance and fluorescence values for the oxidized and reduced forms of alamarBlue?
Example absorbance values are given in Table 3a and example fluorescent values are give in Table 3b. Since these readings can vary depending on the type of media used, a range of powdered media was used. Powdered media was obtained from Sigma and prepared according to their instructions. All media contained phenol red. pH was adjusted to 7.4 with 1N HCI or 1N NaOH. alamarBlue was added to both media which was then split into 2 samples. One sample of both media was autoclaved for 15 minutes to produce the reduced form. The media were dispensed into a flat bottom Dynatech plate (100 µl/well).
Table 3a. Absorbance values for oxidized/reduced forms of alamarBlue for commonly used culture media at different wavelengths.
| Powdered Media | Sigma Product Code | Wavelength (nm) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 540 | 570 | 600 | 630 | ||||||
| OX | RED |
OX |
RED | OX | RED | OX | RED | ||
| BME EBSS | B9638 | 0.61 | 1.207 | 0.853 | 1.502 | 0.845 | 0.244 | 0.261 | 0.177 |
| BME HBS | B9763 | 0.468 | 1.087 | 0.705 | 1.403 | 0.817 | 0.154 | 0.254 | 0.097 |
| McCoy's 5A | M4892 | 0.52 | 1.133 | 0.74 | 1.421 | 0.756 | 0.25 | 0.236 | 0.183 |
| MEM EBSS | M0268 | 0.582 | 1.186 | 0.819 | 1.483 | 0.82 | 0.235 | 0.252 | 0.168 |
| MEM HBSS | M4642 | 0.48 | 1.066 | 0.713 | 1.383 | 0.811 | 0.145 | 0.251 | 0.088 |
| Nut Mix F-10 | N6635 | 0.361 | 0.784 | 0.583 | 1.117 | 0.798 | 0.138 | 0.248 | 0.091 |
| Nut Mix F-12 | N6760 | 0.374 | 0.796 | 0.604 | 1.135 | 0.822 | 0.137 | 0.255 | 0.085 |
| RPMI 1640 | R6504 | 0.431 | 0.928 | 0.659 | 1.25 | 0.795 | 0.161 | 0.248 | 0.101 |
Table 3b. Fluorescence values for oxidized/reduced forms of alamarBlue for commonly used culture media.
| Powdered Media | Sigma Product Code | Fluorescence Units | |
|---|---|---|---|
| Oxidized | Reduced | ||
| BME EBSS | B9638 | 1,926 | 55,676 |
| BME HBS | B9763 | 3,840 | 60,256 |
| McCoy's 5A | M4892 | 2,640 | 50,545 |
| MEM EBSS | M0268 | 2,377 | 54,493 |
| MEM HBSS | M4642 | 4,194 | 59,202 |
| Nut Mix F-10 | N6635 | 2,472 | 70,092 |
| Nut Mix F-12 | N6760 | 5,232 | 68,132 |
| RPMI 1640 | R6504 | 6,472 | 58,796 |
Note: Measurements were taken on a Cambridge Technology, Inc. (Watertown, MA) Model 7620 Microplate. Fluorometer-settings were: bottom reading, light source setting 12, no max AFU; excitation, 530 nm; emission, 580 nm, gain/16.
References
- Breinholt V and Larsen JC (1998). Detection of Weak Estrogenic Flavonoids Using a Recombinant Yeast Strain and a Modified MCF7 Cell Proliferation Assay. Chem Res Toxicol. 11, 622-629.
- De Fries R and Mitsuhashi M (1995). Quantification of mitogen induced human lymphocyte proliferation: Comparison of alamarblue assay to 3h‐thymidine incorporation assay. Journal of clinical laboratory analysts. 9, 89-95.
- Goegan P et al. (1995). Effects of serum protein and colloid on the alamarBlue assay in cell cultures Toxicol In Vitro 9, 257-266.
- Page B et al. (1993). A new Fluorometric Assay for Cytotoxicity Measurements In Vitro. Int J Oncology 3, 473-476.
- Qureshi MH et al. (2001). Neonatal T Cells in an Adult Lung Environment Are Competent to Resolve Pneumocystis carinii Pneumonia. J Immunol 166, 5,704-5,711.
- Simms JR et al. (2000). Use of Herpes Simplex Virus (HSV) Type 1 ISCOMS 703 Vaccine for Prophylactic and Therapeutic Treatment of Primary and Recurrent HSV-2 Infection in Guinea Pigs. The Journal of Infectious Diseases, 181, 1240-1248.



