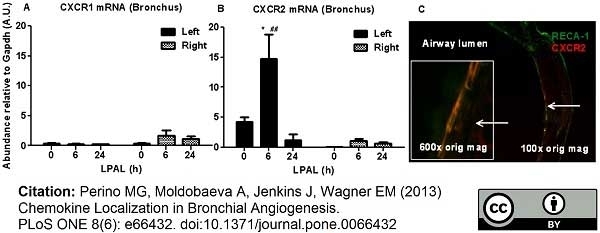
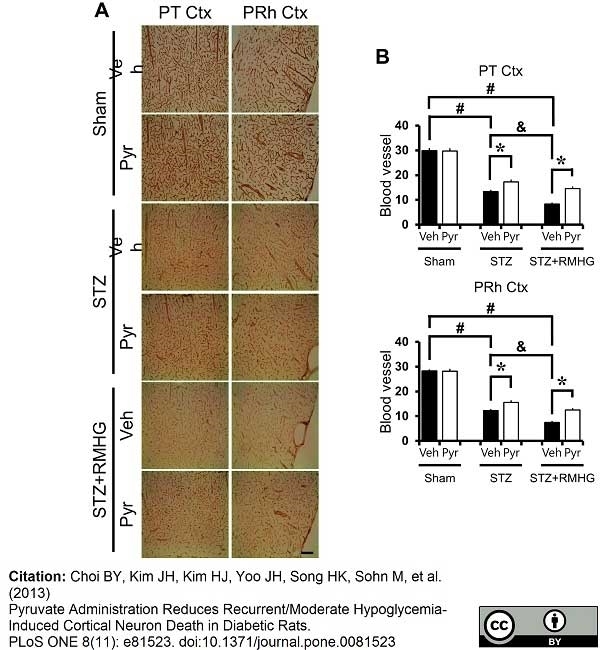
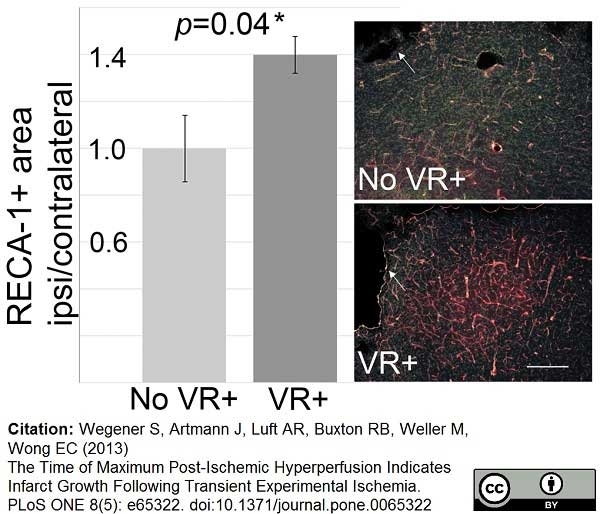
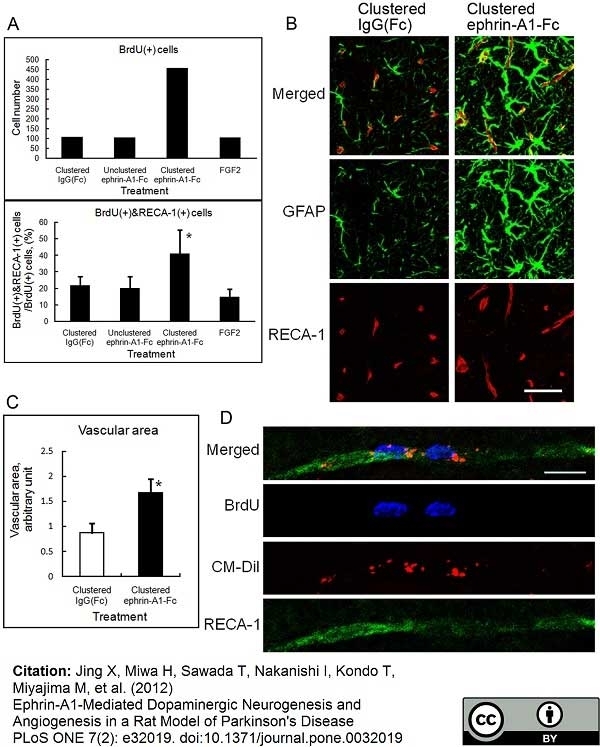
RECA-1 antibody | HIS52
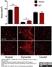
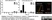

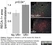
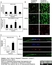
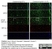
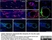


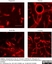
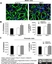
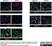
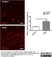
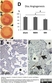



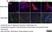


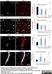
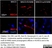

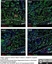
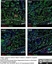










Mouse anti Rat RECA-1
- Product Type
- Monoclonal Antibody
- Clone
- HIS52
- Isotype
- IgG1
- Specificity
- RECA-1
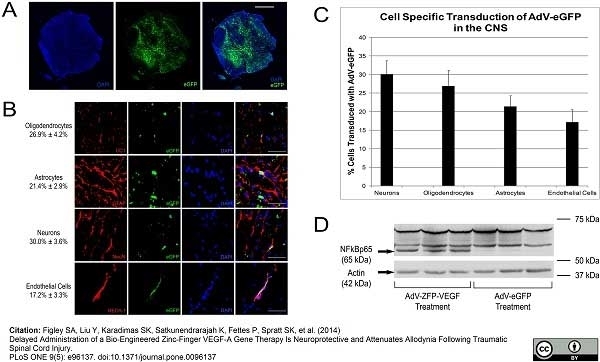
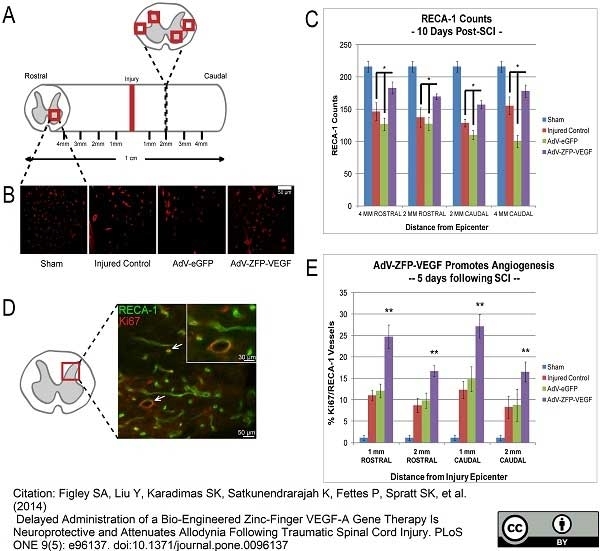
| Mouse anti Rat RECA-1 antibody, clone HIS52 recognizes RECA-1, a cell surface antigen which is expressed by all rat endothelial cells. The endothelium is the thin layer of cells that line the interior surface of blood vessels, forming an interface between circulating blood in the lumen and the rest of the vessel wall. Endothelial cells line the entire circulatory system, from the heart to the smallest capillary. |
- Target Species
- Rat
- Species Cross-Reactivity
-
Target Species Cross Reactivity Goat Chicken Guinea Pig Sheep Mouse Rabbit Pig - N.B. Antibody reactivity and working conditions may vary between species.
- Product Form
- Purified IgG - liquid
- Preparation
- Purified IgG prepared by affinity chromatography on Protein G from tissue culture supernatant
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
- <0.1% Sodium Azide (NaN3)
- Immunogen
- Stromal cells from rat lymph node.
- Approx. Protein Concentrations
- IgG concentration 0.5 mg/ml
- Fusion Partners
- Spleen cells from immunized mice were fused with cells of the SP2/0 mouse myeloma cell line.
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Immunofluorescence | |||
| Immunohistology - Frozen |
References for RECA-1 antibody
-
Duijvestijn, A.M. et al. (1992) Antibodies defining rat endothelial cells: RECA-1, a pan-endothelial cell-specific monoclonal antibody.
Lab Invest. 66 (4): 459-66. -
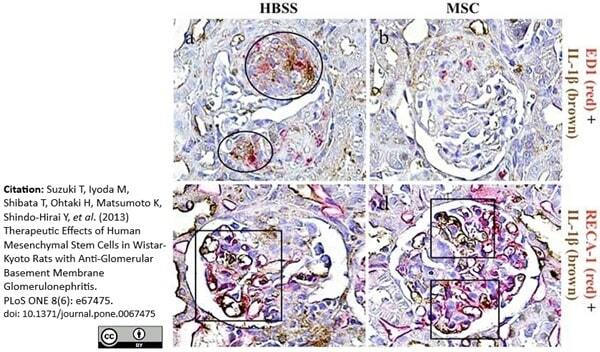
Wada, Y. et al. (2002) Impairment of vascular regeneration precedes progressive glomerulosclerosis in anti-Thy 1 glomerulonephritis.
Kidney Int. 61 (2): 432-43. -
Simard, M. et al. (2003) Signaling at the gliovascular interface.
J Neurosci. 23: 9254-62. -
Tikkanen, J.M. et al. (2006) Role of platelet-derived growth factor and vascular endothelial growth factor in obliterative airway disease.
Am J Respir Crit Care Med. 174: 1145-52. -
Konno, T. et al. (2007) Pregnancy in the brown Norway rat: a model for investigating the genetics of placentation.
Biol Reprod. 76 (4): 709-18. -
Alam, S.M. et al. (2008) Decidual cells produce a heparin-binding prolactin family cytokine with putative intrauterine regulatory actions.
J Biol Chem. 283 (27): 18957-68. -
Valable, S. et al. (2009) MRI assessment of hemodynamic effects of angiopoietin-2 overexpression in a brain tumor model.
Neuro Oncol. 11: 488-502. -
Bexell, D. et al. (2009) Bone marrow multipotent mesenchymal stroma cells act as pericyte-like migratory vehicles in experimental gliomas.
Mol Ther. 17: 183-90.
View The Latest Product References
-
Cattaruzza, F. et al. (2009) Endothelin-converting enzyme 1 promotes re-sensitization of neurokinin 1 receptor-dependent neurogenic inflammation.
Br J Pharmacol. 156: 730-9. -
March, S. et al. (2009) Microenvironmental regulation of the sinusoidal endothelial cell phenotype in vitro.
Hepatology. 50: 920-8. -
Benton RL et al. (2009) Transcriptional activation of endothelial cells by TGFβ coincides with acute microvascular plasticity following focal spinal cord ischaemia/reperfusion injury.
ASN Neuro. 1 (3): pii: e00015. -
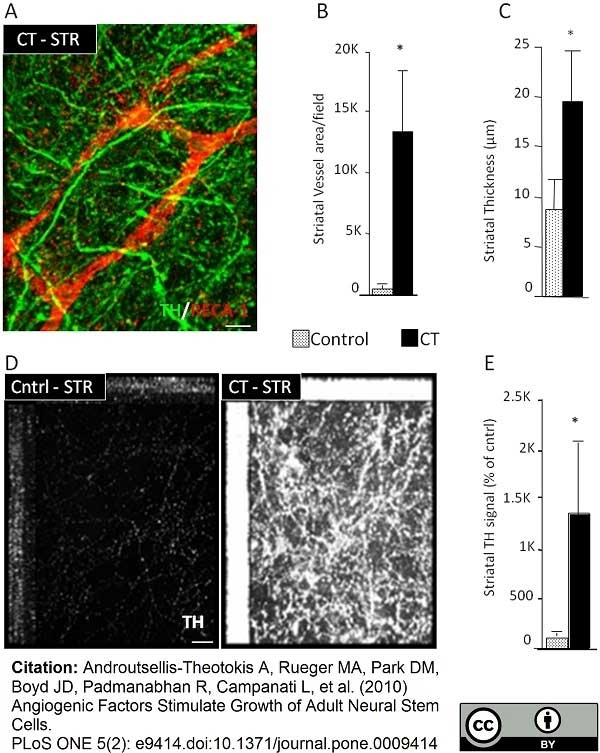
Androutsellis-Theotokis, A. et al. (2010) Angiogenic factors stimulate growth of adult neural stem cells.
PLoS One. 5 (2): e9414. -
Schödel, J. et al. (2010) Factor inhibiting HIF limits the expression of hypoxia-inducible genes in podocytes and distal tubular cells.
Kidney Int. 78 (9): 857-67. -
Szmydynger-Chodobska, J. et al. (2011) Multiple sites of vasopressin synthesis in the injured brain.
J Cereb Blood Flow Metab. 31: 47-51. -
Hamdi, H. et al. (2011) Epicardial adipose stem cell sheets results in greater post-infarction survival than intramyocardial injections.
Cardiovasc Res. 91 (3): 483-91. -
Morin-Brureau, M. et al. (2011) Epileptiform activity induces vascular remodeling and zonula occludens 1 downregulation in organotypic hippocampal cultures: role of VEGF signaling pathways.
J Neurosci. 31: 10677-88 -
Hawthorne, A.L. et al. (2011) The unusual response of serotonergic neurons after CNS Injury: lack of axonal dieback and enhanced sprouting within the inhibitory environment of the glial scar.
J Neurosci. 31: 5605-16. -
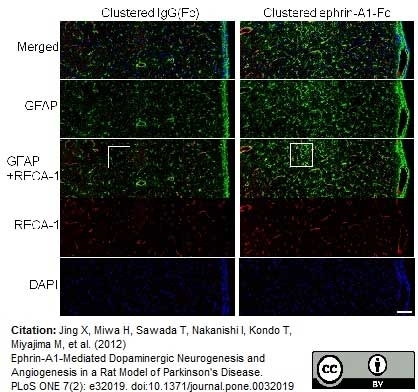
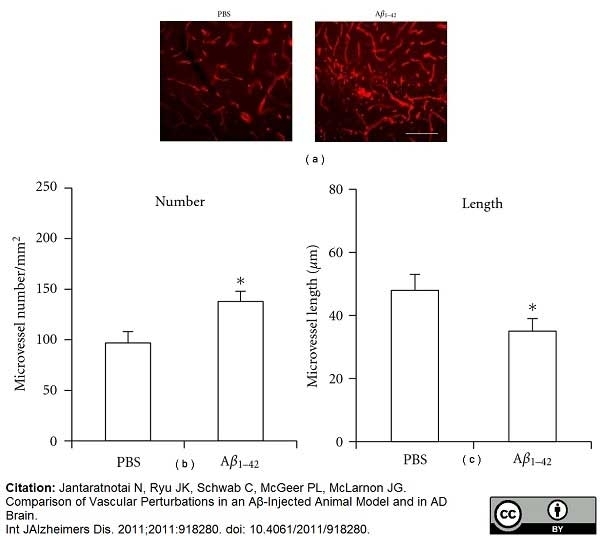
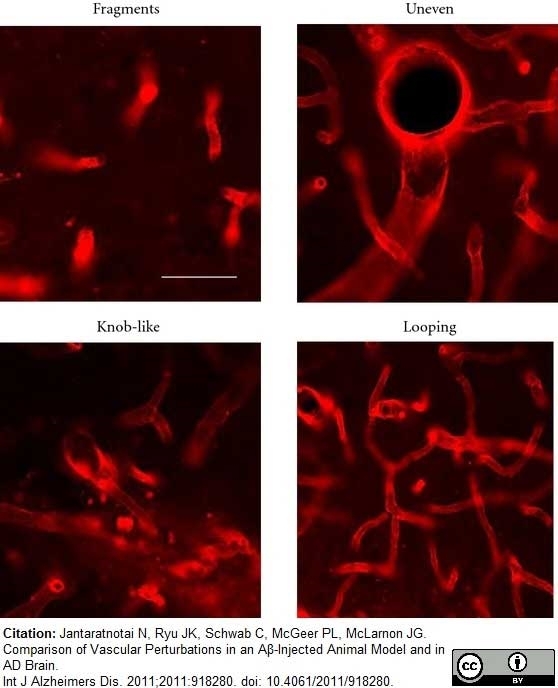
Jantaratnotai, N. et al. (2011) Comparison of Vascular Perturbations in an Aβ-Injected Animal Model and in AD Brain.
Int J Alzheimers Dis. 2011: 918280. -
Miya, M. et al. (2012) Age-related decline in label-retaining tubular cells: implication for reduced regenerative capacity after injury in the aging kidney.
Am J Physiol Renal Physiol. 302: F694-702. -
Nakamura, K. et al. (2014) Soluble thrombomodulin attenuates sinusoidal obstruction syndrome in rat through suppression of high mobility group box 1.
Liver Int. 34 (10): 1473-87. -
Mitkari, B. et al. (2014) Human bone marrow mesenchymal stem/stromal cells produce efficient localization in the brain and enhanced angiogenesis after intra-arterial delivery in rats with cerebral ischemia, but this is not translated to behavioral recovery.
Behav Brain Res. 259: 50-9. -
Wong, K. et al. (2015) Restoration of sensory dysfunction following peripheral nerve injury by the polysaccharide from culinary and medicinal mushroom, Hericium erinaceus. (Bull.: Fr.) Pers. through its neuroregenerative action
Food Sci Technol. 35 (4): 712-21. -
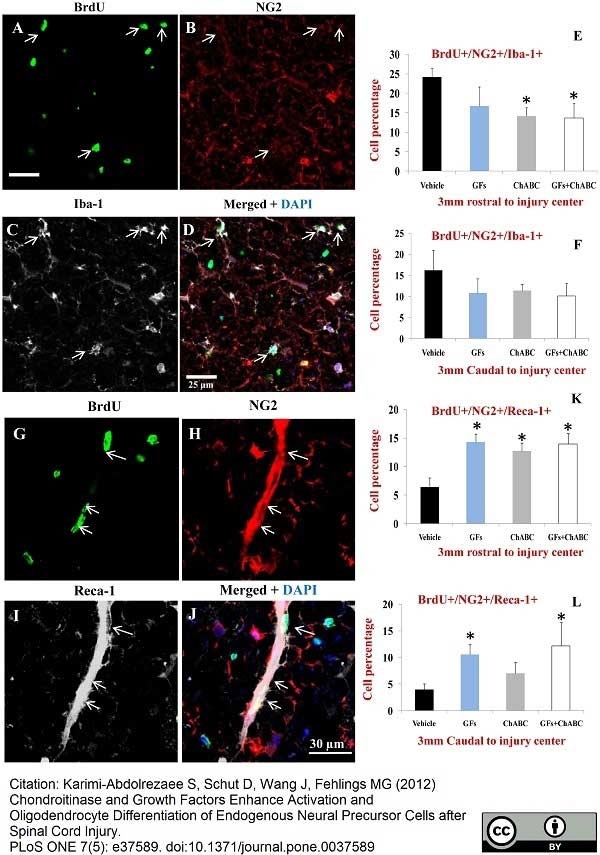
Hu, J. et al. (2015) 3D angioarchitecture changes after spinal cord injury in rats using synchrotron radiation phase-contrast tomography.
Spinal Cord. 53 (8): 585-90. -
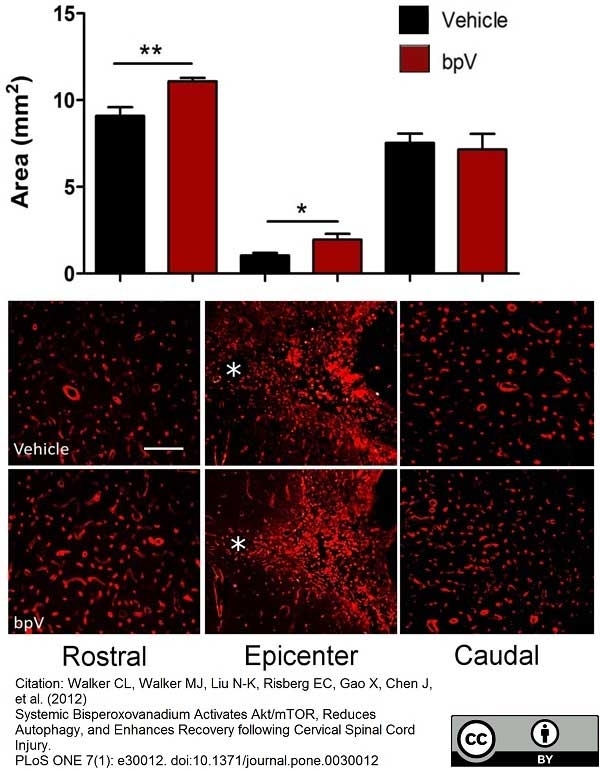
Walker, C.L. et al. (2015) Biphasic bisperoxovanadium administration and Schwann cell transplantation for repair after cervical contusive spinal cord injury.
Exp Neurol. 264: 163-72. -
Ramessur Chandran, S. et al. (2015) Spleen tyrosine kinase contributes to acute renal allograft rejection in the rat.
Int J Exp Pathol. 96 (1): 54-62. -
Van Slooten, A.R. et al. (2015) L-NIO as a novel mechanism for inducing focal cerebral ischemia in the adult rat brain.
J Neurosci Methods. 245: 44-57. -
Nakahara, T. et al. (2015) Structural and functional changes in retinal vasculature induced by retinal ischemia-reperfusion in rats.
Exp Eye Res. 135: 134-45. -
Wang, L. et al. (2015) Lobe-specific expression of phosphodiesterase 5 in rat prostate.
Urology. 85 (3): 703.e7-13. -
Garbelli, R. et al. (2015) PDGFRβ(+) cells in human and experimental neuro-vascular dysplasia and seizures.
Neuroscience. 306: 18-27. -
Syrjälä, S.O. et al. (2015) Donor Heart Treatment With COMP-Ang1 Limits Ischemia-Reperfusion Injury and Rejection of Cardiac Allografts.
Am J Transplant. 15 (8): 2075-84. -
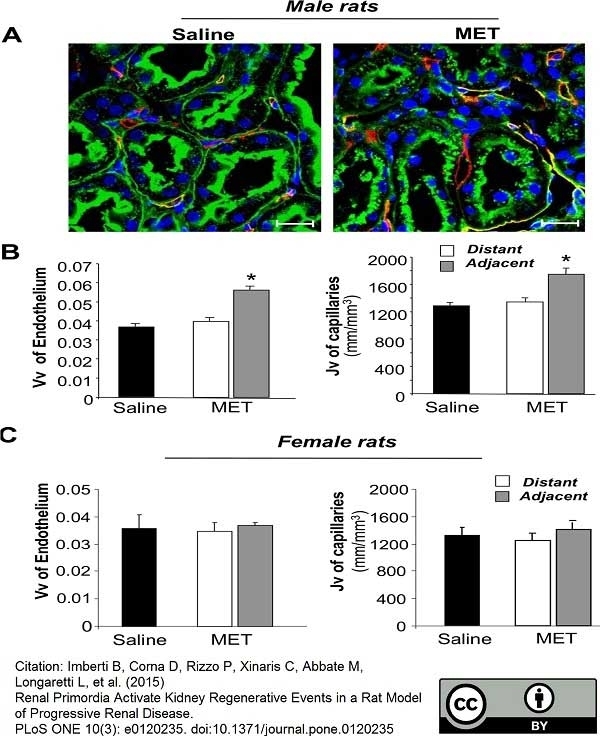
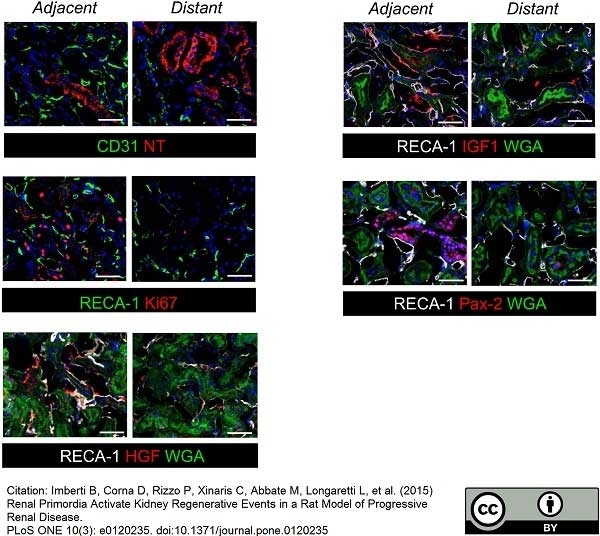
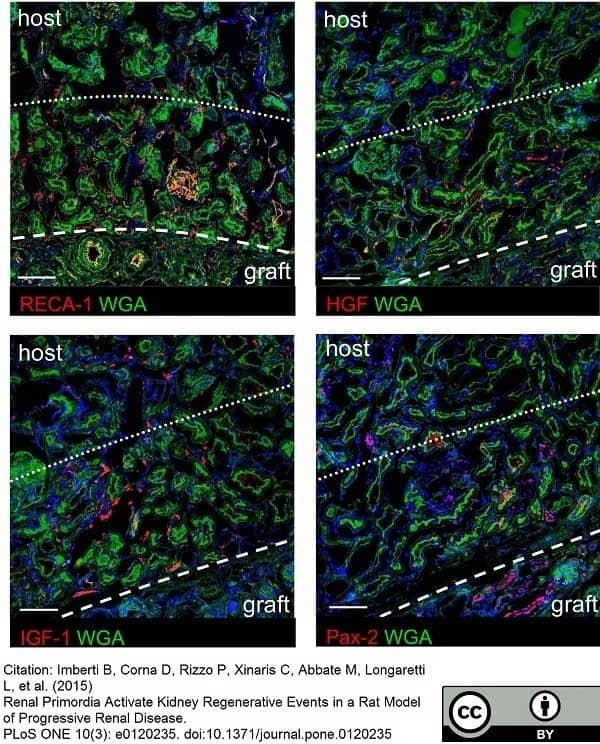
Imberti, B. et al. (2015) Renal Primordia Activate Kidney Regenerative Events in a Rat Model of Progressive Renal Disease.
PLoS One. 10 (3): e0120235. -
Cantaluppi V et al. (2015) Endothelial progenitor cell-derived extracellular vesicles protect from complement-mediated mesangial injury in experimental anti-Thy1.1 glomerulonephritis.
Nephrol Dial Transplant. 30 (3): 410-22. -
Valiente-Soriano, F.J. et al. (2015) BDNF rescues RGCs but not ipRGCs in ocular hypertensive albino rat retinas.
Invest Ophthalmol Vis Sci. pii: IOVS-15-16454. -
Matsushita T et al. (2015) Diffuse and persistent blood-spinal cord barrier disruption after contusive spinal cord injury rapidly recovers following intravenous infusion of bone marrow mesenchymal stem cells.
Exp Neurol. 267: 152-64. -
Wang, J. et al. (2016) Nafamostat mesilate protects against acute cerebral ischemia via blood-brain barrier protection.
Neuropharmacology. 105: 398-410. -
Nakano, A. et al. (2016) Short-term treatment with VEGF receptor inhibitors induces retinopathy of prematurity-like abnormal vascular growth in neonatal rats.
Exp Eye Res. 143: 120-31. -
Nozawa-Inoue, K. et al. (2016) Contribution of synovial lining cells to synovial vascularization of the rat temporomandibular joint.
J Anat. 228 (3): 520-9. -
Cutiongco, M.F. et al. (2016) Planar and tubular patterning of micro and nano-topographies on poly(vinyl alcohol) hydrogel for improved endothelial cell responses.
Biomaterials. 84: 184-95. -
Alves-Sampaio, A. et al. (2016) Biofunctionalized PEDOT-coated microfibers for the treatment of spinal cord injury.
Biomaterials. 89: 98-113. -
Zhang, W. et al. (2016) Omega-3 polyunsaturated fatty acids mitigate blood-brain barrier disruption after hypoxic-ischemic brain injury.
Neurobiol Dis. 91: 37-46. -
Wakamatsu, A. et al. (2016) Role of calcineurin (CN) in kidney glomerular podocyte: CN inhibitor ameliorated proteinuria by inhibiting the redistribution of CN at the slit diaphragm.
Physiol Rep. 4 (6): pii: e12679. -
Liberini, C.G. et al. (2016) The satiating hormone amylin enhances neurogenesis in the area postrema of adult rats.
Mol Metab. 5 (10): 834-43. -
Badner A et al. (2016) Early Intravenous Delivery of Human Brain Stromal Cells Modulates Systemic Inflammation and Leads to Vasoprotection in Traumatic Spinal Cord Injury.
Stem Cells Transl Med. 5 (8): 991-1003. -
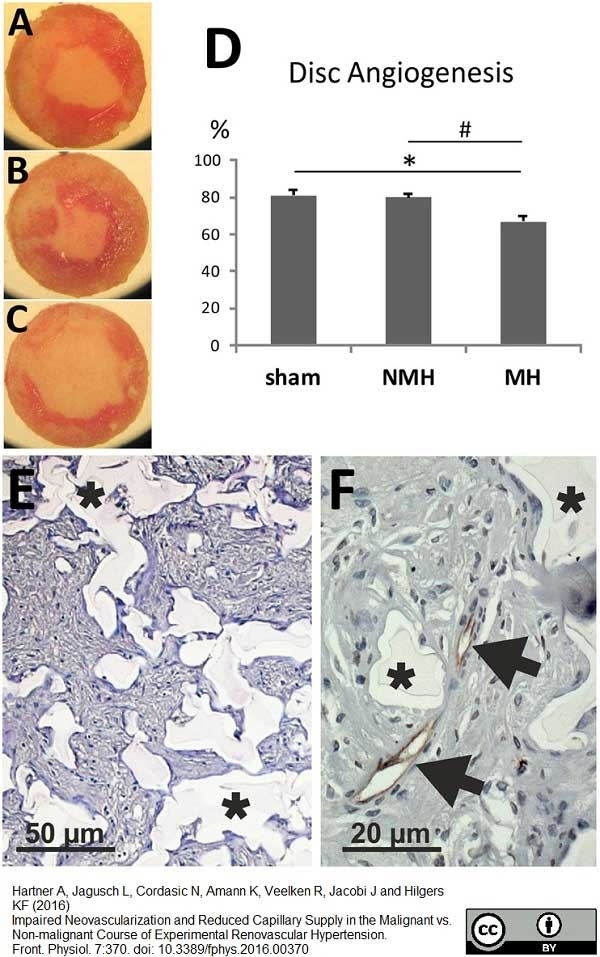
Hartner, A. et al. (2016) Impaired Neovascularization and Reduced Capillary Supply in the Malignant vs. Non-malignant Course of Experimental Renovascular Hypertension.
Front Physiol. 7: 370. -
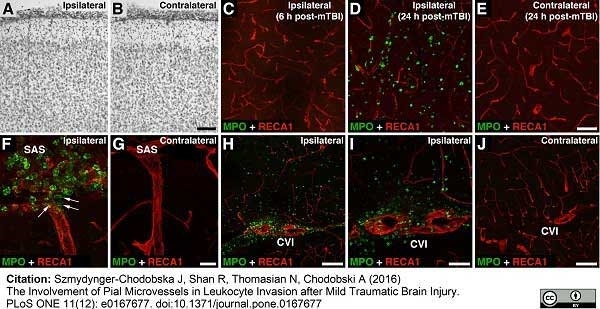
Szmydynger-Chodobska, J. et al. (2016) The Involvement of Pial Microvessels in Leukocyte Invasion after Mild Traumatic Brain Injury.
PLoS One. 11 (12): e0167677. -
Morita, T. et al. (2016) Intravenous infusion of mesenchymal stem cells promotes functional recovery in a model of chronic spinal cord injury.
Neuroscience. 335: 221-31. -
Takahashi, Y. et al. (2017) Rituximab protects podocytes and exerts anti-proteinuric effects in rat adriamycin-induced nephropathy independent of B-lymphocytes.
Nephrology (Carlton). 22 (1): 49-57. -
Okumura, S. et al. (2017) Liver graft preservation using perfluorocarbon improves the outcomes of simulated donation after cardiac death liver transplantation in rats.
Liver Transpl. 23 (9): 1171-85. -
Raissadati, A. et al. (2017) Vascular Endothelial Growth Factor-B Overexpressing Hearts Are Not Protected From Transplant-Associated Ischemia-Reperfusion Injury.
Exp Clin Transplant. 15 (2): 203-12. -
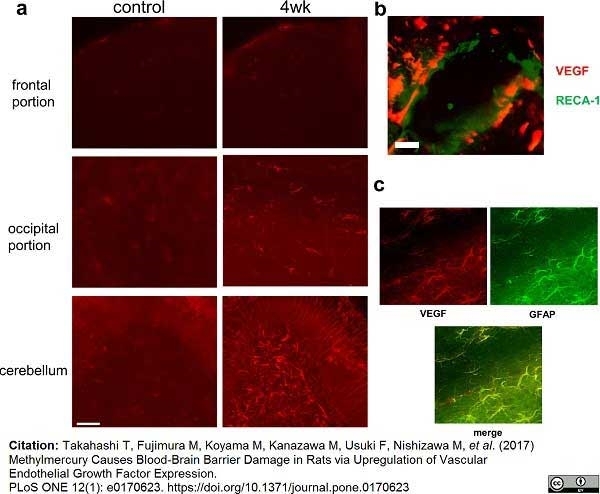
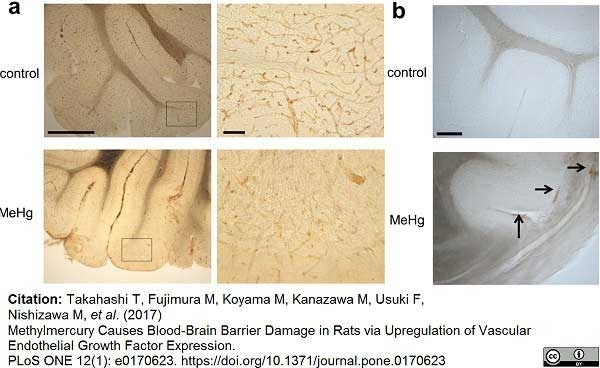
Takahashi, T. et al. (2017) Methylmercury Causes Blood-Brain Barrier Damage in Rats via Upregulation of Vascular Endothelial Growth Factor Expression.
PLoS One. 12 (1): e0170623. -
Becchi, S. et al. (2017) Inhibition of semicarbazide-sensitive amine oxidase/vascular adhesion protein-1 reduces lipopolysaccharide-induced neuroinflammation.
Br J Pharmacol. 174 (14): 2302-17. -
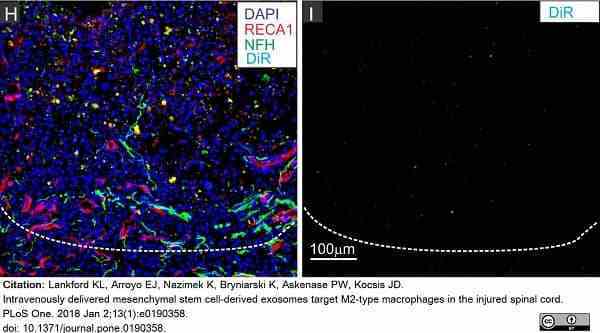
Lankford, K.L. et al. (2018) Intravenously delivered mesenchymal stem cell-derived exosomes target M2-type macrophages in the injured spinal cord.
PLoS One. 13 (1): e0190358. -
Harrell, C.S. et al. (2018) High-fructose diet during adolescent development increases neuroinflammation and depressive-like behavior without exacerbating outcomes after stroke.
Brain Behav Immun. 73: 340-351. -
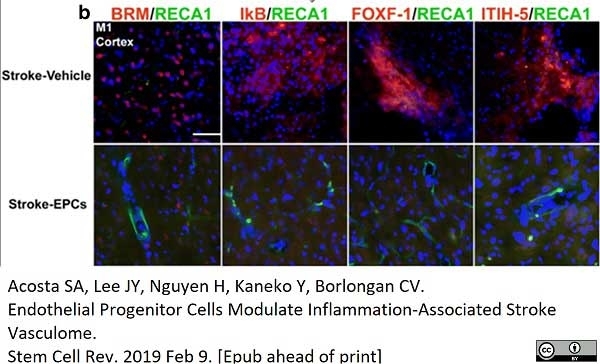
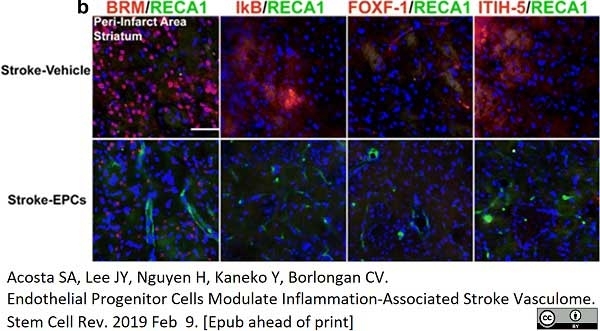
Acosta, S.A. et al. (2019) Endothelial Progenitor Cells Modulate Inflammation-Associated Stroke Vasculome.
Stem Cell Rev Rep. 15 (2): 256-75. -
Nakazaki, M. et al. (2019) Intravenous infusion of mesenchymal stem cells improves impaired cognitive function in a cerebral small vessel disease model.
Neuroscience. 408: 361-77. -
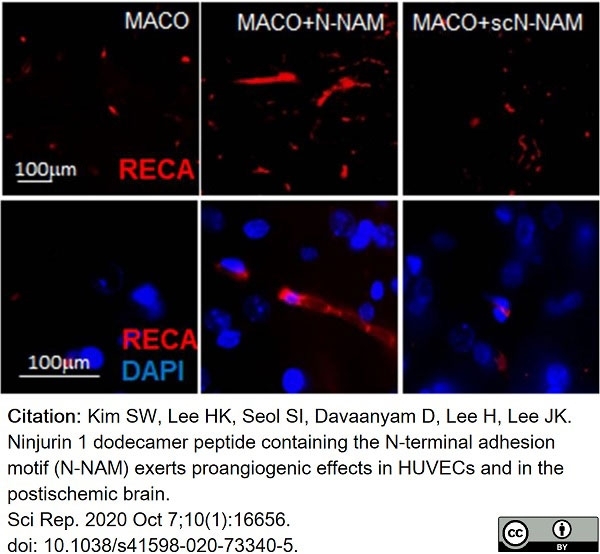
Kim, S.W. et al. (2020) Ninjurin 1 dodecamer peptide containing the N-terminal adhesion motif (N-NAM) exerts proangiogenic effects in HUVECs and in the postischemic brain.
Sci Rep. 10 (1): 16656. -
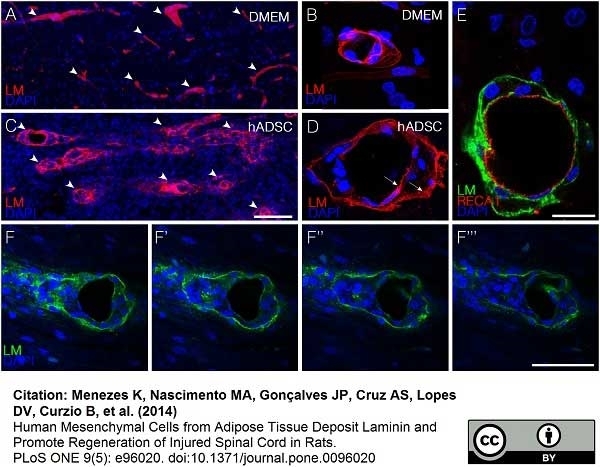
Menezes, K. et al. (2020) Human mesenchymal stromal/stem cells recruit resident pericytes and induce blood vessels maturation to repair experimental spinal cord injury in rats.
Sci Rep. 10 (1): 19604. -
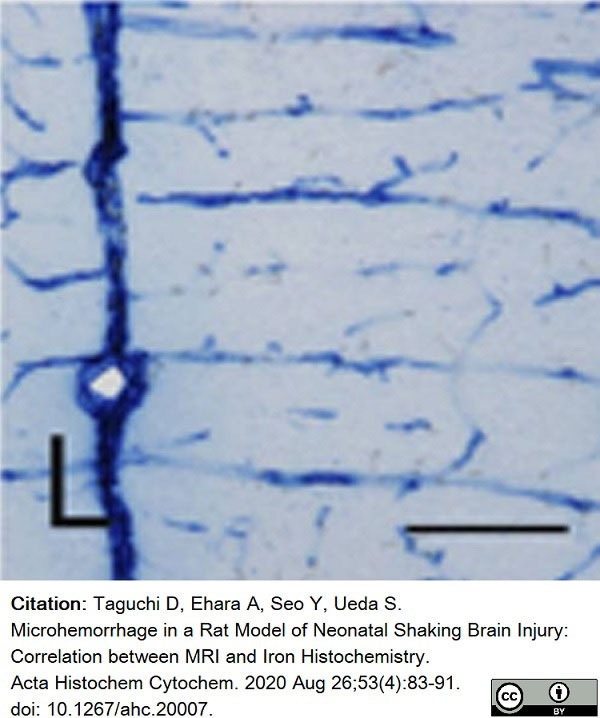
Taguchi, D. et al. (2020) Microhemorrhage in a Rat Model of Neonatal Shaking Brain Injury: Correlation between MRI and Iron Histochemistry.
Acta Histochem Cytochem. 53 (4): 83-91. -
Nakano, A. et al. (2020) Changes in components of the neurovascular unit in the retina in a rat model of retinopathy of prematurity.
Cell Tissue Res. 379 (3): 473-86. -
van Vliet, E.A. et al. (2020) Long-lasting blood-brain barrier dysfunction and neuroinflammation after traumatic brain injury.
Neurobiol Dis. 145: 105080. -
Takeuchi, H. et al. (2021) The efficacy of combining a vascularized biogenic conduit and a decellularized nerve graft in the treatment of peripheral nerve defects: An experimental study using the rat sciatic nerve defect model.
Microsurgery. Dec 25 [Epub ahead of print]. -
Mori, A. et al. (2021) Impairment of endothelium-dependent vasodilator function of retinal blood vessels in adult rats with a history of retinopathy of prematurity
J Pharmacol Sci.146 (4): 233-43. -
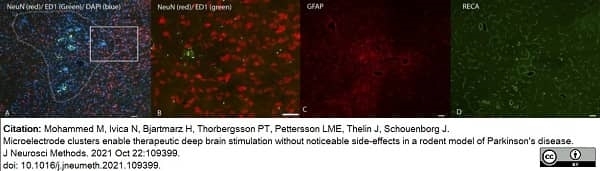
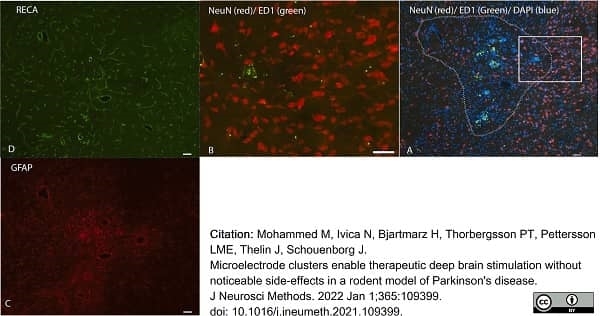
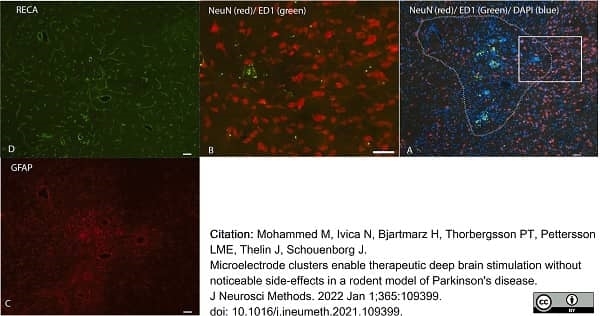
Mohammed, M. et al. (2021) Microelectrode clusters enable therapeutic deep brain stimulation without noticeable side-effects in a rodent model of Parkinson's disease.
J Neurosci Methods. 2021: 109399. -
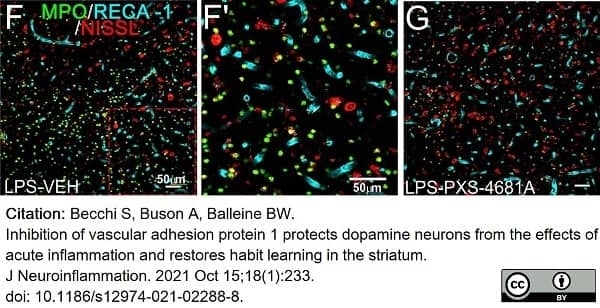
Becchi, S. et al. (2021) Inhibition of vascular adhesion protein 1 protects dopamine neurons from the effects of acute inflammation and restores habit learning in the striatum.
J Neuroinflammation. 18 (1): 233. -
Liu, X. et al. (2022) A novel peptide ligand-coated nano-siRNA-lipoplex technology for kidney targeted gene therapy.
Am J Transl Res. 14 (10): 7362-77. -
Mohammed, M. et al. (2022) Microelectrode clusters enable therapeutic deep brain stimulation without noticeable side-effects in a rodent model of Parkinson's disease.
J Neurosci Methods. 365: 109399. -
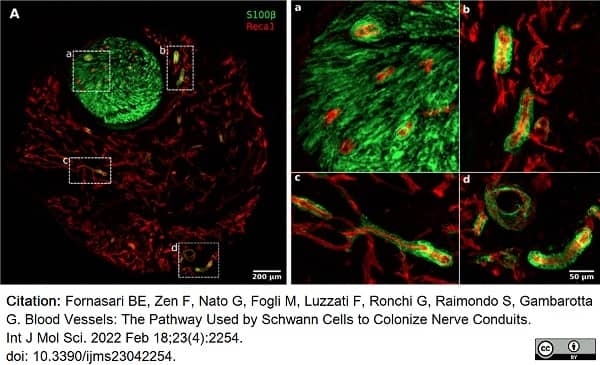
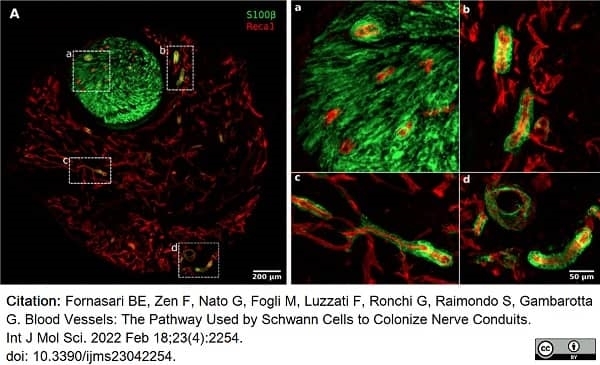
Fornasari, B.E. et al. (2022) Blood Vessels: The Pathway Used by Schwann Cells to Colonize Nerve Conduits.
Int J Mol Sci. 23 (4): 2254. -
Grandizoli, P.S. et al. (2024) Tau Phosphorylation Patterns in the Rat Cerebral Cortex After Traumatic Brain Injury and Sodium Selenate Effects: An Epibios4rx Project 2 Study.
J Neurotrauma. 41 (1-2): 222-43. -
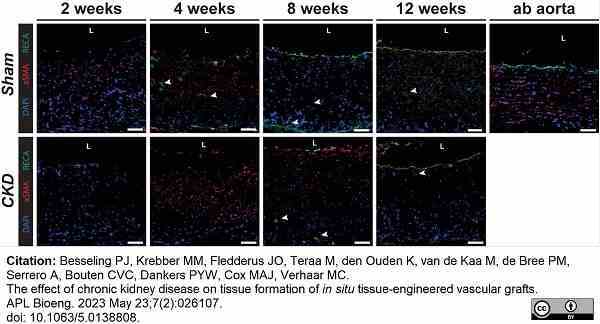
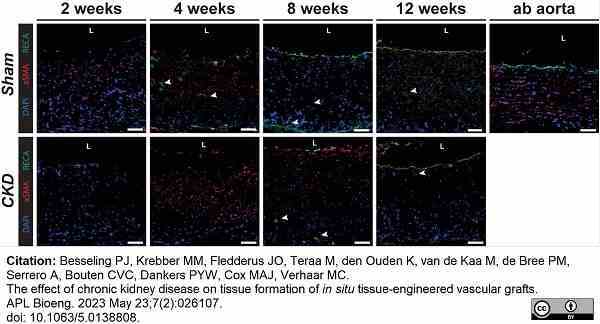
Besseling, P.J. et al. (2023) The effect of chronic kidney disease on tissue formation of in situ tissue-engineered vascular grafts.
APL Bioeng. 7 (2): 026107. -
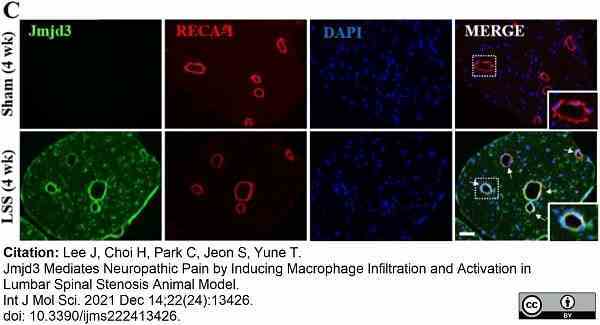
Lee, J. et al. (2021) Jmjd3 Mediates Neuropathic Pain by Inducing Macrophage Infiltration and Activation in Lumbar Spinal Stenosis Animal Model.
Int J Mol Sci. 22 (24): 13426. -
Seo, Y. et al. (2024) Optimal timing for drug delivery into the hippocampus by focused ultrasound: A comparison of hydrophilic and lipophilic compounds
Heliyon. : e29480. -
David, B.T. et al. (2023) Temporary induction of hypoxic adaptations by preconditioning fails to enhance Schwann cell transplant survival after spinal cord injury.
Glia. 71 (3): 648-66.
- RRID
- AB_323297
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Rat ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up