SLA Class I antibody | JM1E3

Mouse anti Pig SLA Class I
- Product Type
- Monoclonal Antibody
- Clone
- JM1E3
- Isotype
- IgG1
- Specificity
- SLA Class I
| Mouse anti Pig SLA Class I antibody, clone JM1E3 recognizes a monomorphic epitope expressed by porcine MHC class I molecules (SLA - 1). SLA - 1 is expressed by all nucleated porcine cells, but not on erythrocytes. This antibody has also been shown to cross-react with human MHC Class I, including HLA-E. (Galiani et al. 2002) The major histocompatibility complex (MHC) is a cluster of genes that are important in the immune response to infections. In pigs, this is referred to as the swine leukocyte antigen (SLA) region. Mouse anti pig SLA class I, clone JM1E3 has been reported to block the interaction of MHC Class I antigens with inhibitory NK cell receptors (Galiani et al. 2002). |
- Target Species
- Pig
- Species Cross-Reactivity
-
Target Species Cross Reactivity Human - N.B. Antibody reactivity and working conditions may vary between species.
- Product Form
- Purified IgG - liquid
- Preparation
- Purified IgG prepared by affinity chromatography on Protein A from tissue culture supernatant
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
- 0.09% sodium azide (NaN3)
- Carrier Free
- Yes
- Immunogen
- Porcine peripheral blood mononuclear cells.
- Approx. Protein Concentrations
- IgG concentration 1.0 mg/ml
- Fusion Partners
- Spleen cells from immunised BALB/c mice were fused with cells of the mouse SP2/0 - Ag14 myeloma cell line.
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Flow Cytometry | 1/50 | 1/100 | |
| Immunofluorescence | |||
| Immunoprecipitation |
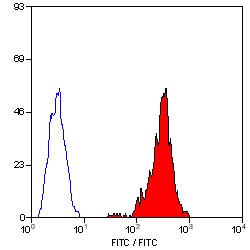
- Flow Cytometry
- Use 10μl of the suggested working dilution to label 106 cells in 100μl
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Mouse IgG1 Negative Control | MCA928 | F | 100 Tests |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Mouse IgG1 Negative Control | ||||||
References for SLA Class I antibody
-
Galiani, D. et al.. (2002) A new monoclonal antibody (JM1E3) specific for porcine SLA Class I antigen recognises HLA Class I antigens and interferes with HLA recognition by human NK inhibitory receptors.
In Leucocyte Typing VII. Edited by Mason. D. et al.. Oxford University Press pp 437-39. -
Park, J.Y. et al. (2008) Characterization of interaction between porcine reproductive and respiratory syndrome virus and porcine dendritic cells.
J Microbiol Biotechnol. 18: 1709-16. -
Jeong, H.J. et al. (2010) Comparative measurement of cell-mediated immune responses of swine to the M
and N proteins of porcine reproductive and respiratory syndrome virus.
Clin Vaccine Immunol. 17: 503-12. -
Ding, G. et al. (2010) Suppression of T cell proliferation by root apical papilla stem cells in vitro.
Cells Tissues Organs. 191: 357-64. -
Hurtado, C. et al. (2011) The African swine fever virus lectin EP153R modulates the surface membrane expression of MHC class I antigens.
Arch Virol. 156: 219-34. -
Van Parys, A. et al. (2012) Salmonella Typhimurium induces SPI-1 and SPI-2 regulated and strain dependent downregulation of MHC II expression on porcine alveolar macrophages.
Vet Res. 43: 52. -
Löndt, B.Z. et al. (2013) Enhanced infectivity of H5N1 highly pathogenic avian influenza (HPAI) virus in pig ex vivo respiratory tract organ cultures following adaptation by in vitro passage.
Virus Res. 178(2):383-91. -
Park, K.M. et al. (2013) Generation of porcine induced pluripotent stem cells and evaluation of their major histocompatibility complex protein expression in vitro.
Vet Res Commun. 37 (4): 293-301.
View The Latest Product References
-
Suarez-Pinzon, W. et al. (2015) A Novel Protocol for Culturing Adult Porcine Islets for Transplantation in Type 1 Diabetic Patients
Minn Acad Sci J Student Res.3: 1-11. -
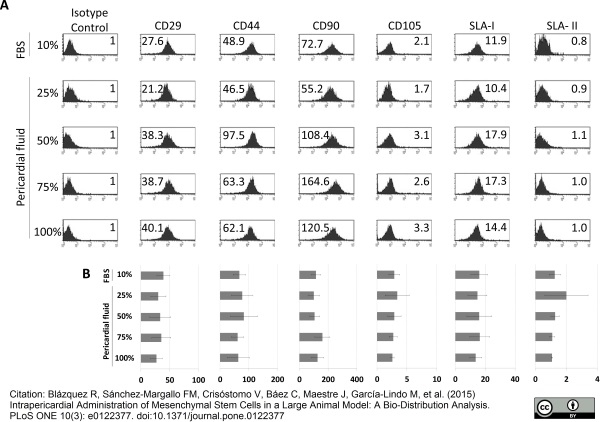
Blázquez, R. et al. (2015) Intrapericardial administration of mesenchymal stem cells in a large animal model: a bio-distribution analysis.
PLoS One. 10 (3): e0122377. -
Richmond, O. et al. (2015) PD-L1 expression is increased in monocyte derived dendritic cells in response to porcine circovirus type 2 and porcine reproductive and respiratory syndrome virus infections.
Vet Immunol Immunopathol. 168 (1-2): 24-9. -
Iwase H et al. (2015) Initial in vivo experience of pig artery patch transplantation in baboons using mutant MHC (CIITA-DN) pigs.
Transpl Immunol. 32 (2): 99-108. -
Rayat, G.R. et al. (2016) First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes - Chapter 3: Porcine islet product manufacturing and release testing criteria.
Xenotransplantation. 23 (1): 38-45. -
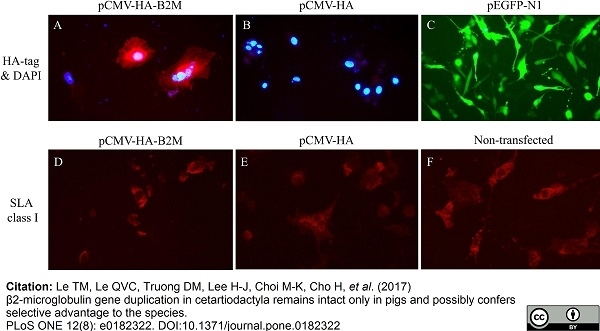
Le, T.M. et al. (2017) β2-microglobulin gene duplication in cetartiodactyla remains intact only in pigs and possibly confers selective advantage to the species.
PLoS One. 12 (8): e0182322. -
Linard, C. et al. (2018) Autologous Bone Marrow Mesenchymal Stem Cells Improve the Quality and Stability of Vascularized Flap Surgery of Irradiated Skin in Pigs.
Stem Cells Transl Med. 7 (8): 569-582. -
Arenal, Á. et al. (2022) Effects of Cardiac Stem Cell on Postinfarction Arrhythmogenic Substrate.
Int J Mol Sci. 23 (24): 16211.
Further Reading
-
Piriou-Guzylack, L. (2008) Membrane markers of the immune cells in swine: an update.
Vet Res. 39: 54.
- UniProt
- O19244
MCA2261GA
If you cannot find the batch/lot you are looking for please contact our technical support team for assistance.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Pig ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up