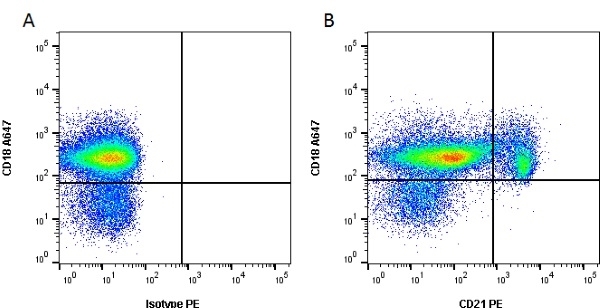
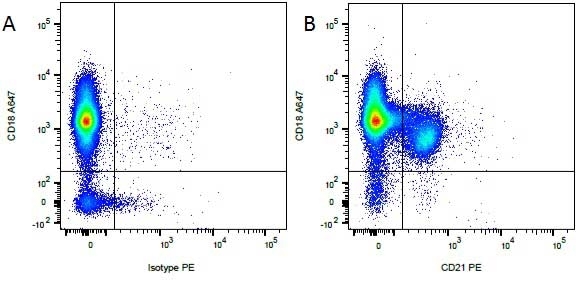
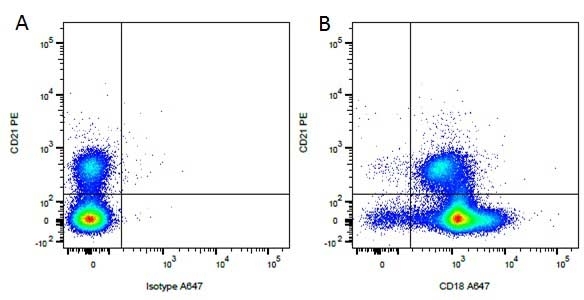
CD21 antibody | CA2.1D6












Mouse anti Canine CD21
- Product Type
- Monoclonal Antibody
- Clone
- CA2.1D6
- Isotype
- IgG1
- Specificity
- CD21
| Mouse anti Canine CD21 antibody, clone CA2.1D6 recognizes canine CD21, also known as Complement receptor type 2. CD21 is a cell surface antigen expressed by canine B lymphocytes. The antigen recognized may be the canine homologue of human CD21, but this has not been fully confirmed. Mouse anti Canine CD21 antibody , clone CA2.1D6 also recognizes the CD21 antigen in Felids. Expression in cats is analogous to that seen in dogs with strong expression on lymphocytes, in a manner mutually exclusive with expression of CD4 or CD8. Mouse anti Canine CD21 antibody, clone CA2.1D6 immunoprecipitates a ~145 kDa protein from feline lymphocytes, similar to the protein immunoprecipitated by the antibody from canine lymphocytes (Dean et al. 1996). |
- Target Species
- Dog
- Species Cross-Reactivity
-
Target Species Cross Reactivity Horse Cat Raccoon - N.B. Antibody reactivity and working conditions may vary between species.
- Product Form
- Purified IgG - liquid
- Preparation
- Purified IgG prepared by affinity chromatography on Protein A from tissue culture supernatant
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
- 0.09% sodium azide (NaN3)
- Approx. Protein Concentrations
- IgG concentration 1.0 mg/ml
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Flow Cytometry | 1/100 | ||
| Immunohistology - Frozen 1 | |||
| Immunohistology - Paraffin | |||
| Immunoprecipitation |
- 1The epitope recognised by this antibody is reported to be sensitive to formaldehyde fixation and tissue processing. Bio-Rad recommends the use of acetone fixation for frozen sections.
- Flow Cytometry
- Use 10μl of the suggested working dilution to label 106 cells or 100μl whole blood
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Mouse IgG1 Negative Control | MCA928 | F | 100 Tests |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Mouse IgG1 Negative Control | ||||||
References for CD21 antibody
-
Cobbold, S. & Metcalfe, S. (1994) Monoclonal antibodies that define canine homologues of human CD antigens: summary of the First International Canine Leukocyte Antigen Workshop (CLAW).
Tissue Antigens. 43 (3): 137-54. -
Dean, G.A. et al. (1996) Proviral burden and infection kinetics of feline immunodeficiency virus in lymphocyte subsets of blood and lymph node.
J Virol. 70 (8): 5165-9. -
Brodersen, R. et al. (1998) Analysis of the immunological cross reactivities of 213 well characterized monoclonal antibodies with specificities against various leucocyte surface antigens of human and 11 animal species.
Vet Immunol Immunopathol. 64 (1): 1-13. -
Hsiao, Y.W. et al. (2004) Tumor-infiltrating lymphocyte secretion of IL-6 antagonizes tumor-derived TGF-beta 1 and restores the lymphokine-activated killing activity.
J Immunol. 172: 1508-14. -
Horn, P.A. et al. (2004) Efficient lentiviral gene transfer to canine repopulating cells using an overnight transduction protocol.
Blood. 103: 3710-6. -
Faldyna, M. et al. (2004) Lymphocyte subsets in synovial fluid from clinically healthy joints of dogs.
Acta Vet. Brno 73: 73-8. -
Jubala, C.M. et al. (2005) CD20 expression in normal canine B cells and in canine non-Hodgkin lymphoma.
Vet Pathol. 42: 468-76. -
Yuasa, K. et al. (2007) Injection of a recombinant AAV serotype 2 into canine skeletal muscles evokes strong immune responses against transgene products.
Gene Ther. 14: 1249-60.
View The Latest Product References
-
Wang, Y.S. et al. (2007) Characterization of canine monocyte-derived dendritic cells with phenotypic and functional differentiation.
Can J Vet Res. 71: 165-74. -
Huang, Y.C. et al. (2008) CD5-low expression lymphocytes in canine peripheral blood show characteristics of natural killer cells.
J Leukoc Biol. 84: 1501-10. -
Reggeti, F. et al. (2008) CD134 and CXCR4 expression corresponds to feline immunodeficiency virus infection of lymphocytes, macrophages and dendritic cells.
J Gen Virol. 89: 277-87. -
Lankford, S. et al. (2008) Cloning of feline FOXP3 and detection of expression in CD4+CD25+ regulatory T cells.
Vet Immunol Immunopathol. 122: 159-66. -
Mortarino, M. et al. (2009) ZAP-70 and Syk expression in canine lymphoid cells and preliminary results on leukaemia cases.
Vet Immunol Immunopathol. 128: 395-401. -
Estrela-Lima, A. et al. (2010) Immunophenotypic features of tumor infiltrating lymphocytes from mammary carcinomas in female dogs associated with prognostic factors and survival rates.
BMC Cancer. 10: 256. -
Bund, D. et al. (2010) Canine-DCs using different serum-free methods as an approach to provide an animal-model for immunotherapeutic strategies.
Cell Immunol. 263: 88-98. -
Araujo, M.S.S. et al. (2011) Immunological changes in canine peripheral blood leukocytes triggered by immunization with first or second generation vaccines against canine visceral leishmaniasis.
Vet Immunol Immunopathol. 141: 64-75. -
Gaurnier-Hausser, A. et al. (2011) NEMO-Binding Domain Peptide Inhibits Constitutive NF-{kappa}B Activity and Reduces Tumor Burden in a Canine Model of Relapsed, Refractory Diffuse Large B-Cell Lymphoma.
Clin Cancer Res. 17: 4661-71. -
Mitchell, L. et al. (2012) Induction of remission results in spontaneous enhancement of anti-tumor cytotoxic T-lymphocyte activity in dogs with B cell lymphoma.
Vet Immunol Immunopathol. 145 (3-4): 597-603. -
Maiolini, A. et al. (2012) Toll-like receptors 4 and 9 are responsible for the maintenance of the inflammatory reaction in canine steroid-responsive meningitis-arteritis, a large animal model for neutrophilic meningitis.
J Neuroinflammation. 9: 226. -
Cave, N.J. et al. (2012) Systemic effects of periodontal disease in cats.
Vet Q. 32: 131-44. -
Aricò, A. et al. (2013) The role of vascular endothelial growth factor and matrix metalloproteinases in canine lymphoma: in vivo and in vitro study.
BMC Vet Res. 9: 94. -
Michael, H.T. et al. (2013) Isolation and characterization of canine natural killer cells.
Vet Immunol Immunopathol. 155 (3): 211-7. -
Aresu, L. et al. (2014) VEGF and MMP-9: biomarkers for canine lymphoma.
Vet Comp Oncol. 12: 29-36. -
Gelain, M.E. et al. (2014) CD44 in canine leukemia: analysis of mRNA and protein expression in peripheral blood.
Vet Immunol Immunopathol. 159 (1-2): 91-6. -
Lin, S-C. et al. (2014) Immune Characterization of Peripheral Blood Mononuclear cells of the Dogs Restored from Innoculation of Canine Transmissible Venereal Tumor Cells.
Tai Vet J. 40 (04): 181-90. -
Izci C et al. (2015) Clinical and light microscopic studies of the conjunctival tissues of dogs with bilateral keratoconjunctivitis sicca before and after treatment with topical 2% cyclosporine.
Biotech Histochem. 90 (3): 223-30. -
Heinrich, F. et al. (2015) Immunophenotyping of immune cell populations in the raccoon (Procyon lotor).
Vet Immunol Immunopathol. 168 (3-4): 140-6. -
Ledbetter, E.C. et al. (2016) Clinical and immunological assessment of therapeutic immunization with a subunit vaccine for recurrent ocular canine herpesvirus-1 infection in dogs.
Vet Microbiol. 197: 102-10. -
Bonnefont-Rebeix, C. et al. (2016) Characterization of a novel canine T-cell line established from a spontaneously occurring aggressive T-cell lymphoma with large granular cell morphology.
Immunobiology. 221 (1): 12-22. -
Gibbons, N. et al. (2017) Phenotypic heterogeneity of peripheral monocytes in healthy dogs.
Vet Immunol Immunopathol. 190: 26-30. -
Martini, V. et al. (2018) Flow cytometry for feline lymphoma: a retrospective study regarding pre-analytical factors possibly affecting the quality of samples.
J Feline Med Surg. 20 (6): 494-501. -
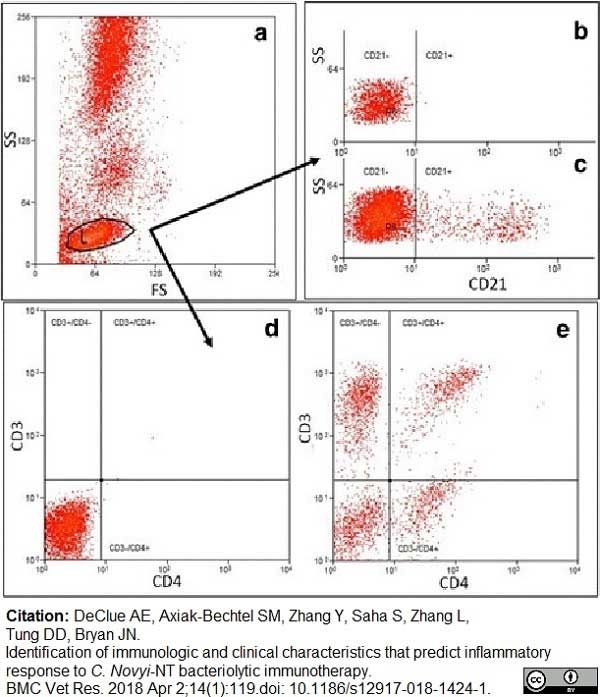
DeClue, A.E. et al. (2018) Identification of immunologic and clinical characteristics that predict inflammatory response to C. Novyi-NT bacteriolytic immunotherapy.
BMC Vet Res. 14 (1): 119. -
DaSilva, A.V.A. et al. (2018) Morphophysiological changes in the splenic extracellular matrix of Leishmania infantum-naturally infected dogs is associated with alterations in lymphoid niches and the CD4+ T cell frequency in spleens.
PLoS Negl Trop Dis. 12 (4): e0006445. -
Schmidli, M.R. et al. (2018) Inflammatory pattern of the infrapatellar fat pad in dogs with canine cruciate ligament disease.
BMC Vet Res. 14 (1): 161. -
Miranda, L.H.M de M. et al. (2018) Co-infection with feline retrovirus is related to changes in immunological parameters of cats with sporotrichosis.
PLoS One. 13 (11): e0207644. -
Shin, N. et al. (2018) INCB040093 Is a Novel PI3Kδ Inhibitor for the Treatment of B Cell Lymphoid Malignancies.
J Pharmacol Exp Ther. 364 (1): 120-30. -
Sato, M. et al. (2018) Prognostic significance of hypermethylation of death-associated protein kinase (DAPK) gene CpG island in dogs with high-grade B-cell lymphoma.
Vet Comp Oncol. 16 (3): 409-15. -
Martini, V. et al. (2019) Prognostic role of non-neoplastic lymphocytes in lymph node aspirates from dogs with diffuse large B-cell lymphoma treated with chemo-immunotherapy.
Res Vet Sci. 125: 130-5. -
Jimbo, S. et al. (2019) Natural and inducible regulatory B cells are widely distributed in ovine lymphoid tissues.
Vet Immunol Immunopathol. 211: 44-8. -
Maeta, N. et al. (2019) Lymphokine-activated killer cell transplantation after anti-cancer treatment in two aged cats.
Open Vet J. 9 (2): 147-50. -
Aguiar-Soares, R.D.O. et al. (2020) Phase I and II Clinical Trial Comparing the LBSap, Leishmune(®), and Leish-Tec(®) Vaccines against Canine Visceral Leishmaniasis.
Vaccines (Basel). 8 (4): 690. -
Wolf-Ringwall, A. et al. (2020) Prospective evaluation of flow cytometric characteristics, histopathologic diagnosis and clinical outcome in dogs with naïve B-cell lymphoma treated with a 19-week CHOP protocol.
Vet Comp Oncol. 18 (3): 342-52. -
Lucassen, A. et al. (2021) A Saccharomyces cerevisiae Fermentation Product (Olimond BB) Alters the Early Response after Influenza Vaccination in Racehorses.
Animals (Basel). 11(9):2726. -
Lee, J. et al. (2021) Canine Natural Killer Cell-Derived Exosomes Exhibit Antitumor Activity in a Mouse Model of Canine Mammary Tumor.
Biomed Res Int. 2021: 6690704. -
Grudzien, M. et al. (2021) A newly established canine NK-type cell line and its cytotoxic properties.
Vet Comp Oncol. 19 (3): 567-77. -
Yang, Y. et al. (2021) Canine Multicentric Large B Cell Lymphoma with Increased Mott Cells Diagnosed by Flow Cytometry
Journal of Veterinary Clinics. 38 (1): 36-40. -
Lee, S.H. et al. (2021) Safety and immunological effects of recombinant canine IL-15 in dogs.
Cytokine. 148: 155599. -
Knebel, A. et al. (2021) Measurement of canine Th17 cells by flow cytometry.
Vet Immunol Immunopathol. 243: 110366. -
Riccardo, F. et al. (2022) Antigen mimicry as an effective strategy to induce CSPG4-targeted immunity in dogs with oral melanoma: a veterinary trial.
J Immunother Cancer. 10 (5): e004007. -
Jaensch, S.M. et al. (2022) Clinicopathologic and immunophenotypic features in dogs with presumptive large granular lymphocyte leukaemia.
Aust Vet J. 100 (11): 527-32. -
Troupel, T. et al. (2022) Generalised idiopathic polymyositis mimicking masticatory myositis in a dog
Veterinary Record Case Reports. 10 (4) [Epub ahead of print]. -
Rotolo, A. et al. (2023) Unedited allogeneic iNKT cells show extended persistence in MHC-mismatched canine recipients.
Cell Rep Med. 4 (10): 101241. -
Townsend, K.S. et al. (2023) Concurrent chronic lymphocytic leukemia and primary hyperparathyroidism in a mule.
J Vet Intern Med. 37 (3): 1250-5. -
Wesolowski, M. et al. (2023) Long-term changes of Th17 and regulatory T cells in peripheral blood of dogs with spinal cord injury after intervertebral disc herniation.
BMC Vet Res. 19 (1): 90. -
Martini, V. et al. (2018) A retrospective study of flow cytometric characterization of suspected extranodal lymphomas in dogs.
J Vet Diagn Invest. 30 (6): 830-6. -
DeClue, A.E. et al. (2020) Transportation and Routine Veterinary Interventions Alter Immune Function in the Dog.
Top Companion Anim Med. 39: 100408. -
Rütgen, B.C. et al. (2022) Composition of lymphocyte subpopulations in normal and mildly reactive peripheral lymph nodes in cats.
J Feline Med Surg. 24 (2): 77-90. -
Cha, S. et al. (2023) Non-B, Non-T Acute Lymphoblastic Leukemia in a Cat
Journal of Veterinary Clinics. 40 (4): 298-302. -
Lee, G.W. et al. (2021) Case Report: Long-Term Survival of a Dog With Chronic Lymphocytic Leukemia Treated With Chlorambucil, Prednisolone, and Imatinib.
Front Vet Sci. 8: 625527. -
Sainz, Á. et al. (2021) Effect of chemically modified tetracycline-8 (CMT-8) on hematology, blood chemistry, cytokines and peripheral blood lymphocyte subsets of healthy dogs.
Res Vet Sci. 136: 200-8. -
Placci, M. et al. (2020) Natural Horse Boarding Vs Traditional Stable: A Comparison of Hormonal, Hematological and Immunological Parameters.
J Appl Anim Welf Sci. 23 (3): 366-77. -
Sheng, R. et al. (2023) Prognostic significance of CD25 expression in dogs with a noninvasive diagnosis of B-cell lymphoma treated with CHOP chemotherapy.
Vet Comp Oncol. 21 (1): 28-35. -
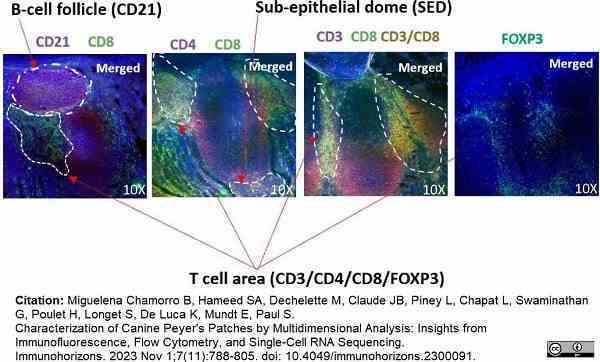
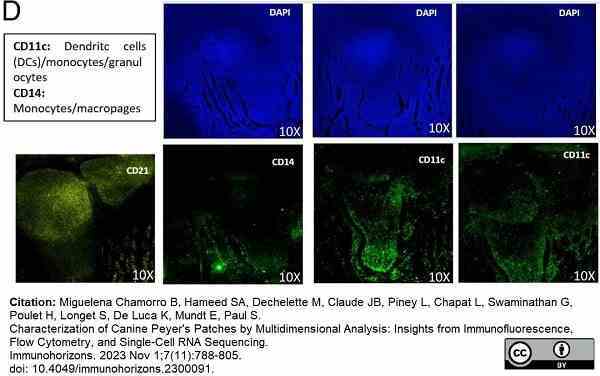
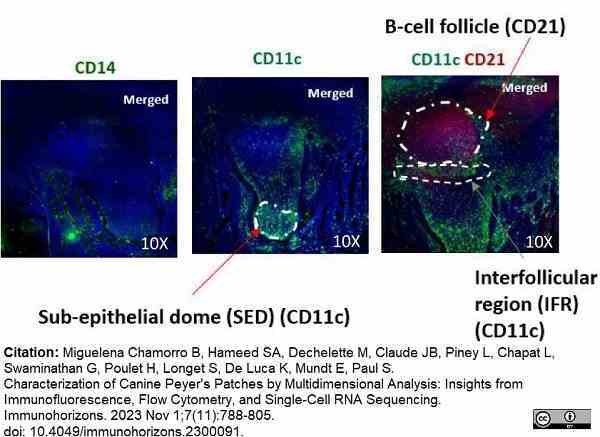
Miguelena Chamorro, B. et al. (2023) Characterization of Canine Peyer's Patches by Multidimensional Analysis: Insights from Immunofluorescence, Flow Cytometry, and Single-Cell RNA Sequencing.
Immunohorizons. 7 (11): 788-805. -
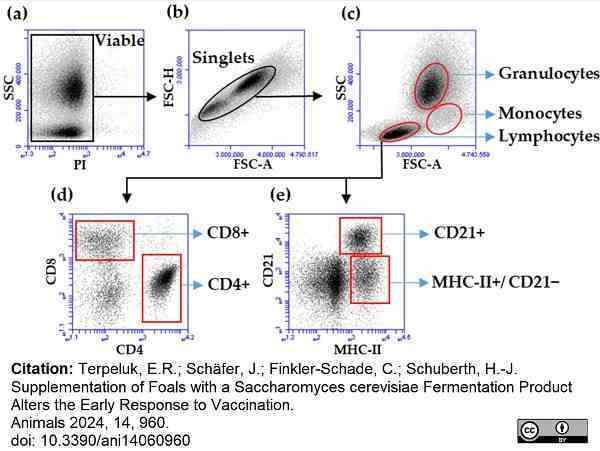
Terpeluk, R.E. et al. (2024) Supplementation of Foals with a Saccharomyces cerevisiae Fermentation Product Alters the Early Response to Vaccination
Animals. 14 (6): 960. -
Mason, N.J. et al. (2021) Development of a fully canine anti-canine CTLA4 monoclonal antibody for comparative translational research in dogs with spontaneous tumors.
MAbs. 13 (1): 2004638. -
Yuan, C. et al. (2024) Effects of Porcine Epidemic Diarrhea Virus Infection on CD21+ B cells Activation
Veterinary Microbiology. : 110087.
- RRID
- AB_323665
MCA1781R
If you cannot find the batch/lot you are looking for please contact our technical support team for assistance.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Dog ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up