Guided Selection Strategies
-
Monoclonal Generation
-
Custom Recombinant Monoclonal Antibody Generation
-
HuCAL® Antibody Generation Process
- Antigens and antigen expression services
- Guided Selection Strategies
- Affinity maturation
- Selection of antibodies based on binding strength
- Extended QC services
- Fab antibody formats and epitope tags
- Conversion of Fab to full immunoglobulin format
- Fab Antibody Production
- Screening and Pair Selection using the Bio-Plex® System
- Flow cytometry antibody screening
-
HuCAL® Antibody Generation Process
-
Custom Recombinant Monoclonal Antibody Generation
s
Simplified sourcing via Scientist.com
s
Custom antibody project inquiry form
A personal, no obligation quotation for a custom monoclonal antibody generation project
s
Contact our custom antibody specialists
Tel: +49 (0) 89 80 90 95 45
Fax: +49 (0) 89 80 90 95 50
Office: Bio-Rad AbD Serotec GmbH, Campus Neuried, Anna-Sigmund-Str. 5, 82061 Neuried, Germany
Selection of antibodies using HuCAL® technology is performed in vitro. This enables greater flexibility for antibody generation than is available with conventional methods based on the immunization of animals. Guided selection involves blocking steps or the use of two or more antigens, and is typically used for the isolation of epitope-specific antibodies or to find antibodies that recognize shared epitopes on different antigens. Additional strategies allow us to select matched pairs for sandwich ELISA, or to alter selection conditions to ensure the antigen is presented to the antibody library in the form in which it will be assayed, e.g. denatured, captured, soluble or masked. Buffer conditions can also be set during antigen-antibody binding to simulate the application-specific experimental conditions.
Epitope-specific and phospho-specific antibodies
We have developed successful strategies to select antibodies from the HuCAL library that are specific for epitopes such as phosphorylation or oxidation sites. Selection is carried out on a synthetic peptide modified at the relevant amino acid. Before selection, all antibodies in the library that bind the non-modified peptide are efficiently blocked with an excess of the non-modified peptide. Further counter-screening and quality control (QC) ensure that the antibody is specific to the modified epitope.
Example 1: Phospho-specific antibodies
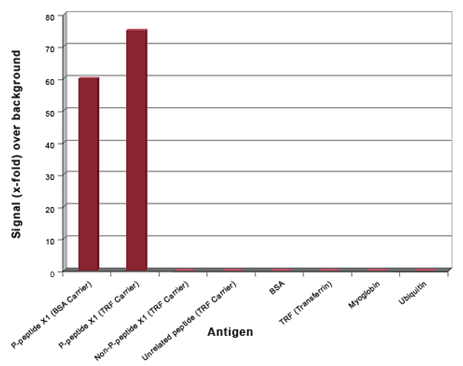
Fig. 1. ELISA QC of a phospho-specific antibody. Phospho-specific antibodies were generated against a 9 amino acid peptide phosphorylated at ser-6 (P-Peptide X1). The library was blocked with the non-phosphorylated peptide, followed by panning against the phosphorylated peptide coupled to BSA and transferrin. ELISA QC shows that the antibody specifically recognizes the phosphorylated peptide and does not react with the non-phosphorylated peptide, an unrelated phosphorylated peptide, or the carrier proteins.
Example 2: Oxidation site-specific antibodies
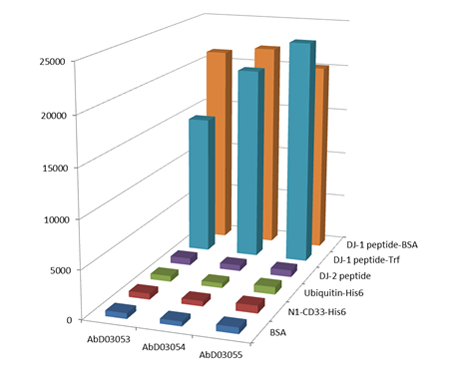
Fig. 2. Antibodies were generated against oxidized DJ-1 protein using a 12 amino acid DJ-1 peptide containing the C106 oxidation site. The library was blocked with the non-oxidized peptide (DJ-2 peptide) and then panned against the oxidized DJ-1 peptide coupled to BSA and transferrin. ELISA QC shows that all 3 selected antibodies specifically recognize the oxidized peptide and do not react with the non-oxidized peptide or the carrier proteins. Antibody HCA024 (clone AbD03055) was shown to bind to full length oxidized DJ-1.
Ooe H et al. (2003). Specific cleavage of DJ-1 under an oxidative condition. Neurosci Lett. 406:165-8
Example 3: Enzyme active site-specific antibodies
Antibodies specific to the active site of a proteinase were isolated from the HuCAL library using a blocking strategy with a mutant enzyme that contained a single point mutation in the active site. Resulting antibodies were inhibitory for the enzyme activity.

Antibodies specific to the West Nile Virus (WNV) NS2B-NS3 proteinase active site were isolated from the HuCAL library using phage display blocking strategies, providing antibody research tools that enabled scientists to carry out inhibition experiments and structural studies.
Antibodies lacking cross-reactivity to closely related proteins
A subtraction strategy can be used to eliminate cross-reacting antibodies from the selection process. Pre-adsorption steps and intelligent counter-screening drive the selection towards the unique epitopes on the antigen protein.
Example 4: Development of an assay to discriminate between native and truncated CXCL10 chemokine

Antibodies were selected that enable differential quantification for two forms of the inflammatory chemokine CXCL10 in biological samples. The antibodies bind specifically to either native CXCL10 or an antagonist form, which is truncated by just two amino acids at the N-terminus.
Anti-idiotypic antibody generation
Special guided selection strategies are used for anti-idiotypic antibody generation. Selection of the antibody is carried out using the monoclonal antibody drug as the antigen in the presence of isotype sub-class matched antibodies as blockers to avoid enrichment of specificities that bind to other regions of the antibody drug, and to ensure idiotope specificity. Additionally, selection performed in the presence of human serum avoids matrix effects in the final assay.
The selection method can be guided to generate anti-idiotypic antibodies with different binding modes and properties. For example, inhibitory antibodies that block the drug binding to its target are ideal for cell based assays and ELISA. Selection of an antibody that binds to an idiotope outside the antigen binding site of the drug results in a non-inhibitory antibody that can be used to detect both free and bound drug in the sample. Unique to Bio-Rad is the capability to isolate rare specificities, such as drug-target complex binders that can be used to quantify bound drug, as opposed to free drug levels.
Antibodies that bind homologous proteins
Alternate selection using two antigens allows identification of antibodies that react with both antigens if they share a common epitope. This strategy is useful for generating cross-species antibodies, for recognizing two isoforms of a protein, or identifying anti-peptide antibodies that also react with the parental protein.
Sandwich ELISA antibody pair selection
Two strategies are typically used for the generation of sandwich pair antibodies. Antibody selection (panning) against the antigen is followed by testing all possible combinations of the selected antibodies as capture and detection antibodies in a sandwich ELISA or bead-based assay.
Standard panning against the antigen is followed by ELISA screening of the purified antibodies for the best capture antibody, which is then used to capture the antigen. A subsequent panning is performed on the antibody-antigen complex.

HuCAL Antibodies Technical Manual - HuCAL Technology
Our guided selection strategies are described in more detail in the 'HuCAL Technology' chapter of our technical manual





