s
Western Blot University
s
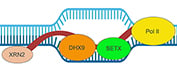
What are R-loops?
s
Cancer Pathway Posters
Compiling key proteins involved in key signaling pathways: cGAS-STING, EGF R, mTOR, NF-kB, and PI3/AKT pathways.
s
Recent Blog
Overview
This protocol describes how to detect phosphorylated proteins by western blotting using Phospho-Specific Validated PrecisionAb Antibodies. We also provide a list of reagents that work optimally with our antibodies.

Protocol: Detection of Phosphorylated Proteins by Western Blotting
Download as PDF
 Webinar: Illuminating the Pathway to Confident Western Blot Detection of Phosphorylated Proteins
Webinar: Illuminating the Pathway to Confident Western Blot Detection of Phosphorylated Proteins
In some cases specific recommendations are provided on product datasheets, and these methods should always be used in conjunction with product and batch specific information provided with each vial. Note that a certain level of technical skill and immunological knowledge is required for the successful design and implementation of these techniques - these are guidelines only and may need to be adjusted for particular applications.
Protocol
Reagents
Clarity Western ECL Substrate Kit (170-5061)
Criterion 4-15% TGX Stain-Free Gels (567-8084)
Dithiothreitol (DTT) (161-0611)
Lambda Protein Phosphatase
Laemmli Sample Buffer (161-0737)
Precision Plus All Blue Standards (161-0373)
Precision Plus Unstained Standards (161-0363)
1x TBS 1% Casein Blocking Buffer (161-0782)
10x TGS Running Buffer (161-0772)
Trans-Blot Turbo RTA Mini/Midi Transfer Kit, LF PVDF (170-4274, 170-4275)
10x Tris-Buffered Saline (TBS) (170-6435)
10x Tris/Glycine/SDS (TGS; running buffer) (161-0772)
10% Tween 20 (161-0781)
Before you start:
- Prepare TBST Wash Buffer (1x TBS with 0.1% Tween 20) using 10% Tween 20, 10x TBS, and deionized water (DI H2O)
- Prepare Casein Tween Blocking Buffer (1x Casein Blocking Buffer + 0.1% Tween 20) using 1x TBS 1% Casein Blocking Buffer and 10% Tween 20
- Prepare Phosphatase Buffer (100 mM NaCl, 50 mM Tris-HCl, 10 mM MgCl2, 1 mM MnCl2, 2 mM DTT pH 7.9 at 25°C)
- Prepare 1x TGS
- Prepare Transfer Buffer (included in Trans-Blot Turbo RTA Mini/Midi Transfer Kit, LF PVDF)
-
Prepare lysates
Methods:
Gel electrophoresis
- Prepare 1x TGS Running Buffer using 10x TGS Running Buffer and DI H2O.
- Remove an 18-well Criterion 4-15% TGX Stain-Free gel from the packaging (ensuring the green comb and the white tape are removed), rinse gel wells with DI H2O to ensure all air bubbles are removed. Place gel into the Criterion Cell and assemble according to the instruction manual. Fill the inner chamber with 1x TGS Running Buffer, ensuring the wells are covered.
-
Mix Precision Plus All Blue Standards and Precision Plus Unstained Standards at a 1:1 ratio and load 10 µl per well into appropriate wells of the gel (see suggested gel layout below). Note that Precision Plus Unstained Standards are required in order to visualize the standards using stain-free technology and ChemiDoc Imaging Systems pre and post transfer.
Learn more about stain-free technology -
Load lysates into appropriate wells of the gel.
Learn more about controls for western blot detection of phosphorylated targets
Table 1. Suggested loading for the gel for Phospho-Specific PrecisionAb Antibodies.
Lane Sample Antibody Blot treatment 1 Empty 2 MW Standard Total Mock 3 Untreated Lysate Total Mock 4 Treated Lysate Total Mock 5 MW standard Total Mock 6 MW standard Total PP Treated 7 Untreated Lysate Total PP Treated 8 Treated Lysate Total PP Treated 9 MW Standard Total PP Treated 10 MW Standard Phospho-specific Mock 11 Untreated Lysate Phospho-specific Mock 12 Treated Lysate Phospho-specific Mock 13 MW Standard Phospho-specific Mock 14 MW Standard Phospho-specific PP treated 15 Untreated Lysate Phospho-specific PP treated 16 Treated Lysate Phospho-specific PP treated 17 MW Standard Phospho-specific PP treated Abbreviations: MW standard, protein MW standard; untreated lysate, lysate not treated to stimulate/preserve phosphorylation of target protein; treated lysate, lysate treated to stimulate/preserve phosphorylation of target protein; mock, blot incubated under same conditions as protein phosphatase (PP) treated except no phosphatase is added; PP treated, blot incubated with lambda PP to dephosphorylate proteins bound to membrane; total, anti-total protein antibody; phospho-specific, phospho-specific PrecisionAb Antibodies.
-
Attach the Criterion Cell to the power pack and set the voltage to 300 V. Run until the dye front reaches the bottom of the gel (approximately 20-25 min).
-
Remove the gel from the cassette. Use gel separators to cut the well “fringe” from the top of the gel and the “foot” from the bottom of the gel. Place the gel in a large blotting box with 1x TGS Running Buffer.
Stain-free image of the membrane and protein transfer
-
UV activation of stain-free gel is required for TPN imaging.
Learn more about how to perform TPN analysis using stain-free technology - Use the Trans-Blot Turbo RTA Midi LF PVDF Transfer Kit which includes the low fluorescence PVDF membrane, required for stain-free imaging of the blot for the total protein normalization (TPN) steps. The use of low fluorescence PVDF rather than normal PVDF or nitrocellulose allows visualization of the fluorescence emitted from the activated tri-halo compounds bound to the tryptophan residues of the proteins on the gel which have now been transferred to the membrane. The use of the high molecular weight protocol is also recommended to ensure efficient transfer of all proteins onto the membrane.
-
Once the transfer is complete, capture a stain-free image of the membrane.
Membrane blocking
- Place the membrane into a large blotting box with 15 ml of Casein Tween Blocking Buffer. Using a pencil, mark the corner of each membrane, so you can distinguish between them later. Incubate for 30 min at room temperature (RT) on a shaker set to 150 rpm.
Phosphatase treatment
- Prepare 20 ml of 1x Phosphatase Buffer per gel, adding 2 µl of 5 M MnCl2 and 10 µl of 2 M DTT per 10 ml of Phosphatase Buffer just before use.
-
Label the chambers of the 6-well mini-strip blotting box with the blot number:
- Chamber 1 Anti-total protein antibody, mock
- Chamber 2 Anti-total protein antibody, PP treated
- Chamber 3 Phospho-specific antibody, mock
-
Chamber 4 Phospho-specific antibody, PP treated
- Add 3 ml of 1x Phosphatase Buffer to each chamber of the blotting box.
- Add 5 µl of DI H2O to each chamber that will contain a membrane not treated with phosphatase (mock).
- Add 5 µl of Lambda Protein Phosphatase (LambdaPP) to each chamber that will contain a membrane to be phosphatase treated.
- Cut the membrane between each set of standard lanes.
- Place each strip into the corresponding chamber of the blotting box and incubate for 2 hr at 37°C on a rocker (1660709EDU) set to 30 rpm.
- Carefully pour off the 1x Phosphatase Buffer from the chambers of the blotting box.
- Add 10 ml TBST Wash Buffer to each chamber and incubate at RT or 5 min on a shaker set to 150 rpm.
-
Pour off the wash solution and repeat for a total of 5 washes.
Western blotting
- Dilute all required primary antibodies to the required working dilution (for Phospho-Specific PrecisionAb range, use dilution 1:1,000) in Casein Tween Blocking Buffer.
- Carefully pour off the final TBST wash from each chamber and add 3 ml of each diluted primary antibody to the appropriate chamber of the blotting box.
- Incubate overnight at 4˚C on a rocker set to 30 rpm.
- The following day, carefully pour off the primary antibody, avoiding chamber-to-chamber contamination.
- Add 10 ml TBST Wash Buffer and incubate at RT for 5 min on a shaker set to 150 rpm.
- Pour off the wash solution and repeat for a total of 5 washes.
- Prepare the appropriate secondary antibody to the required working dilution by diluting in Casein Tween Blocking Buffer.
- Add 3 ml of diluted secondary antibody to the appropriate chamber of the blotting box and incubate for 1 hr at RT on a shaker.
- Carefully pour off the secondary antibody, avoiding chamber to chamber contamination.
- Add 10 ml TBST Wash Buffer to each chamber and incubate at RT for 5 min on a shaker.
-
Pour off the wash solution and repeat for a total of 5 washes.
Chemiluminescent development
- Mix Clarity Western ECL Substrate Kit components at a 1:1 ratio in a clean tube. A total of 1 ml of mixed substrate will be needed for each strip of membrane.
- Pick up the membrane with a pair of clean forceps and gently shake off excess TBST Wash Buffer.
- Assemble both membranes for each antibody (mock and phosphatase treated) on the same sheet protector in the following orientation (from left to right):
a) Mock treated.
b) Phosphatase treated. (This orientation is necessary if you want to perform TPN analysis)
- Gently apply 1 ml of the mixed ECL substrate directly on top of each membrane, covering the membrane as evenly as possible.
- Allow to incubate for 5 min at RT.
Imaging
- Image using the ChemiDoc MP Imager and analyze using ImageLab software.