PrecisionAb Antibodies — Antibodies with Enhanced Validation
Antibodies are one of the most important research tools for biomedical science. In recent years, a number of studies have questioned the reliability of published data, and highlighted how poor-quality antibodies have contributed to a lack of consistent results (Bordeaux et al. 2010, Prinz et al. 2011, Baker and Dolgin 2017).
What Is Antibody Validation?
“Antibody validation is the experimental proof and documentation that a specific antibody is suitable for an intended application or purpose” (Weller 2018). To address this, the International Working Group on Antibody Validation (IWGAV) has suggested broad guidelines for antibody validation (Uhlen et al. 2016). The IWGAV defined five validation pillars that provide a framework for validation testing. These include genetic strategies, orthogonal strategies, independent antibody strategies, expression of tagged proteins, and immunocapture followed by mass spectrometry (MS).
 Bio-Rad’s PrecisionAb Antibody Range
Bio-Rad’s PrecisionAb Antibody Range
At Bio-Rad, we are committed to providing our customers with reliable research tools and are constantly raising our standards for antibody quality. The PrecisionAb Antibody range, rigorously tested in western blotting for specificity and reproducibility, is designed to help researchers produce the highest-quality western blotting data. We are also expanding the applications PrecisionAb Antibodies are tested in to include immunofluorescence (IF) and immunoprecipitation (IP) (Figure 1).
PrecisionAb Antibodies are evaluated against multiple biologically relevant cell lysates and tissues that express the endogenous target (Figure 1). Where relevant, negative controls and other important samples, such as human serum and human or rodent tissue lysates, are included. For each antibody, we carefully review the literature to identify lysates expected to express the target and use this to evaluate the antibody. Western blots are carried out by scientists who scrutinize the data comparing relevant bioinformatic database references against signal-to-background ratio, ensuring that only the best antibodies are selected as PrecisionAb Antibodies.
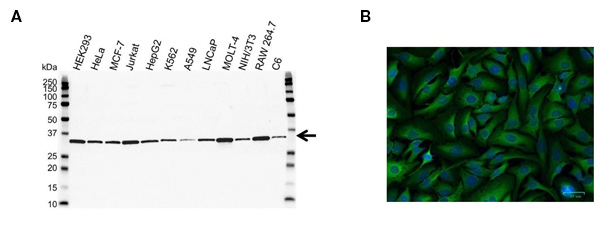
Fig. 1. A, western blot analysis on multiple cell lines probed with PCNA Antibody (VMA00018) arrow points to PCNA (molecular weight 29 kDa). B, HeLa cells stained with 5 µg/ml Mouse Anti-GPI Antibody (VMA00348, green). Hoechst was used as nuclear stain (blue) and imaged with a 20x objective.
Using Bio-Rad’s stain-free imaging technology, we monitor protein loading consistency and transfer efficiency.
Transparent Validation Methods
We understand the importance of transparency in our production and testing processes, guiding customers to the correct reagents for their research needs. For this reason, every antibody comes with in-depth information including details of the immunogen used in its generation, and recommended conditions for suggested applications like western blotting. Our western blots are not cropped to narrow the molecular weight range, so any additional bands and off-target effects are never excluded. Reviews of antibodies and information about publications where the antibody has been used are also provided.
Explore the PrecisionAb Antibodies Range
Knockout and Knockdown Validation
At Bio-Rad, we are taking the necessary steps to enable researchers to perform reliable and reproducible western blotting experiments using our antibodies.
To comply with the IWGAV recommendations, we are also implementing validation methods recommended by the five-pillar validation approach. This includes genetic knockout using CRISPR/CAS9 followed by comparison of target protein levels in knockout versus wild type cells (Figure 2).
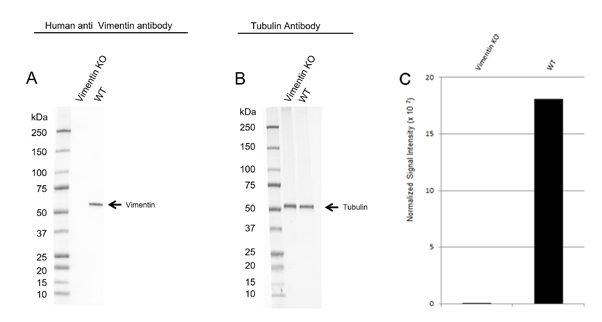
Fig 2. Western blot analysis of vimentin CRISPR knockout HeLa (Vimentin KO) and wild type HeLa (WT) whole cell lysates probed with A, Human Anti-Vimentin Antibody (VMA00008) and B, hFAB Rhodamine Anti-Tubulin Primary Antibody (12004166). C, normalized signal intensity of vimentin from CRISPR knockout and wild type lysates. Signal intensity was normalized to total protein.
Explore the KO Validated PrecisionAb Antibodies Range
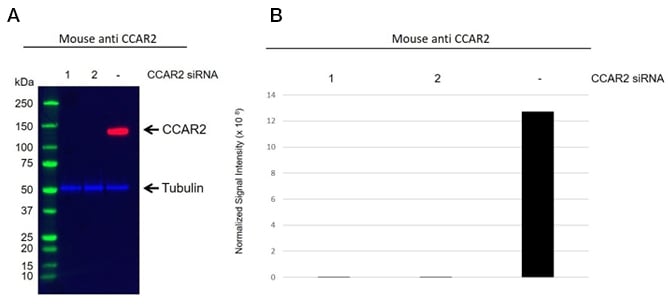
We also perform siRNA knockdown. Figure 3 illustrates the difference between siRNA-treated (Figure 3A lane 1 and 2) and untreated (Figure 3A lane -) cells by a western blot for a specific CCAR2 antibody (VMA00564). We quantify signal intensity for each antibody validated through the siRNA mediated approach (Figure 3B). The antibody reactivity signal in siRNA treated samples is reduced in intensity compared with the control samples proving the antibody’s specificity. The datasheet and validation data provided online for each antibody are batch-specific.
Fig. 3. A, western blot analysis of whole cell lysates prepared from HEK293 separately transfected with two unique siRNA duplexes against CCAR2 (1 & 2), or a scrambled siRNA (-) probed with Mouse Anti-CCAR2 Antibody (VMA00564) and hFAB Rhodamine Anti-Tubulin Primary Antibody (12004166). Visualized on the ChemiDoc MP Imaging System with a 60 sec (chemiluminescent channel) and 10 sec (rhodamine channel) exposure. B, normalized signal intensity of CCAR2 to the tubulin signal.
Explore the KD Validated PrecisionAb Antibodies Range
IP-MS Validation
For antibody target verification, we also implement immunoprecipitation followed by mass spectrometry (IP-MS). First, we optimize immunoprecipitation for the chosen antibody. Next, the IP eluate samples are analyzed by MS to identify peptide sequences from the protein in the sample. In order to verify target specificity, after subtracting the background, we look at the number of unique peptides detected and their protein abundance (total abundance for all peptides from a given protein). Significant proteins are identified by a high number of unique peptides and a high abundance. This validation method demonstrates that the antibody can recognize the cognate antigen expressed at endogenous levels in the cell lysate.
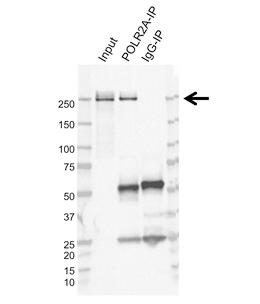
Fig. 4. Immunoprecipitation of POLR2A from K562 whole cell lysate. The input lane is 10% input. The POLR2A-IP lane is the immunoprecipitation with the POLR2A Antibody (VMA00613). The IgG-IP lane is the immunoprecipitation with Mouse Monoclonal IgG (MCA929). Western blot analysis was performed using POLR2A Antibody (VMA00613) followed by detection with HRP Conjugated Goat Anti-Mouse IgG (1705047) and visualized on the ChemiDoc MP Imaging System. Arrow points to POLR2A.
Explore the IP-MS Validated PrecisionAb Antibodies Range
Phosphorylation-Specific Antibody Validation
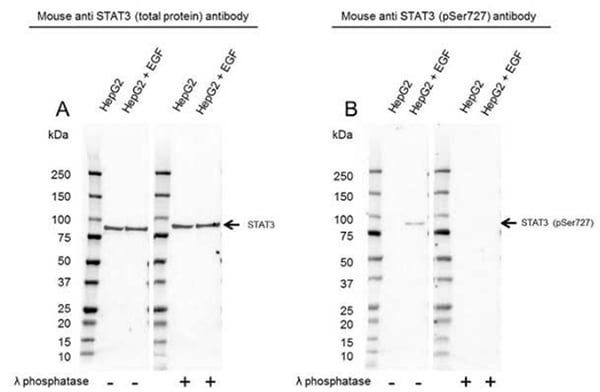
For phosphorylation-specific antibodies, we validate by comparing antibody binding in treated (for kinase activation or phosphatase inhibition) versus untreated cell lysates, and by dephosphorylating proteins on western blot membranes. To showcase the different binding characteristics of phospho-specific and total protein antibodies, Phospho-Specific PrecisionAb Antibodies are compared against total PrecisionAb Antibodies. This enables researchers to assess the impact of different treatments and provides a control recommendation (Figure 5).
Fig.5. Western blot analysis of HepG2 untreated and EGF treated whole cell lysates probed with A, Mouse Anti-STAT3 Antibody (VMA00242) or B, Mouse Anti-STAT3 (pSer727) Antibody (VMA00755), followed by detection with HRP Conjugated Goat Anti-Mouse IgG (1/10,000, STAR207P). Membranes were treated with (+) and without (-) lambda protein phosphatase and visualized on the ChemiDoc MP Imaging System with a A, 52 sec or a B, 300 sec exposure. STAT3 (pSer727) has a molecular weight of 84 kDa.
Explore the Phospho-Specific PrecisionAb Antibodies Range
Select the Right Antibody
Need a little clarity and guidance when it comes to antibody choice?
Our Select the Right Antibody for your Experiments guide runs you through the key considerations when selecting an antibody. Don't forget Bio-Rad also offers unrivaled technical support for those queries that need expert assistance. We are committed to providing you with the products and guidance to help you succeed with your experiments.
Choosing Validated Antibodies for Trustworthy Data
 Learn about the validation methods implemented by Bio-Rad, including CRISPR-Cas9 gene editing, siRNA, and immunoprecipitation followed by mass spectrometry, ensuring the specificity and reliability of our antibodies in your application.
Learn about the validation methods implemented by Bio-Rad, including CRISPR-Cas9 gene editing, siRNA, and immunoprecipitation followed by mass spectrometry, ensuring the specificity and reliability of our antibodies in your application.
Watch the video
References
- Baker M and Dolgin E (2017). Cancer reproducibility project releases first results. Nature 541(7637), 269-270.
- Bordeaux J et al. (2010). Antibody validation. Biotechniques 48(3), 197-209.
- Prinz F et al. (2011). Believe it or not: how much can we rely on published data on potential drug targets? Nat Rev Drug Discov 10(9), 712.
- Uhlen M et al. (2016). A proposal for validation of antibodies. Nature Methods 13, 823-827.
- Weller MG (2018). Ten basic rules of antibody validation. Anal Chem Insights 13:1177390118757462.