CD3 antibody | CD3-12






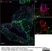
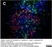





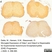

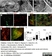
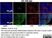



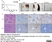
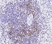













Rat anti Human CD3
- Product Type
- Monoclonal Antibody
- Clone
- CD3-12
- Isotype
- IgG1
- Format
- Purified
- Specificity
- CD3
| Rat anti Human CD3 antibody, clone CD3-12 raised against a peptide representing an invariant cytoplasmic sequence within the CD3ε chain recognizes human CD3ε. CD3 is a multimeric protein complex composed of four distinct polypeptide chains (ε, γ, δ, ζ) that assemble and function as three pairs of dimers (εγ, εδ, ζζ). The CD3 complex serves as a T cell co-receptor that associates non-covalently with the T cell receptor (TCR) (Malissen 2008; Guy and Vignali 2009; Smith-Garvin et al. 2009). CD3 is a defining feature of cells belonging to the T cell lineage and can therefore be used as T cell marker. As Rat anti Human CD3, clone CD3-12 has been raised against an epitope within the epsilon peptide chain, highly conserved among species clone CD3-12 has a very broad species crossreactivity for the CD3 marker. (Jones et al. 1993; Kothlow et al. 2005). |

|
- Target Species
- Human
- Species Cross-Reactivity
-
Target Species Cross Reactivity Bovine Dog Horse Rhesus Monkey Pig Chicken Mouse Duck Koala Harbour Porpoise Alpaca Cynomolgus monkey Spotted Hyena Sea Lion Cat Amazon Parrot Mammals Expected from Sequence Birds Expected from Sequence Raccoon Great horned owl (Bubo virginianus) Bullfrog Xenopus Amphibia Expected from Sequence Rabbit African green monkey - N.B. Antibody reactivity and working conditions may vary between species.
- Product Form
- Purified IgG - liquid
- Preparation
- MCA1477: Purified IgG prepared by affinity chromatography on Protein G from tissue culture supernatant
- MCA1477T: Purified IgG prepared from tissue culture supernatant
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
- 0.09% sodium azide (NaN3)
- Carrier Free
- Yes
- Immunogen
- Synthetic peptide sequence derived from cytoplasmic epitope of CD3 (Glu-Arg-Pro-Pro-Pro-Val-Pro-Asn-Pro-Asp-Tyr-Glu-Pro-Cys) (ERPPPVPNPDYEPC )
- Approx. Protein Concentrations
- IgG concentration 1.0 mg/ml
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
- Acknowledgements
- PrecisionAb is a trademark of Bio-Rad Laboratories
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
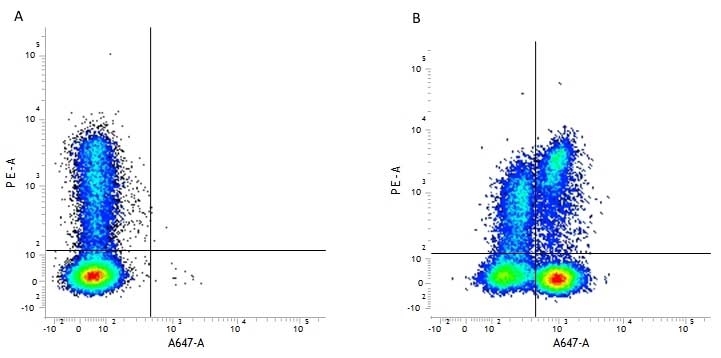
| Flow Cytometry 1 | 1/50 | 1/100 | |
| Immunofluorescence | |||
| Immunohistology - Frozen | 1/100 | ||
| Immunohistology - Paraffin 2 | 1/100 | ||
| Western Blotting |
- 1 Membrane permeabilization is required for this application. The use of Leucoperm (Product Code BUF09) is recommended for this purpose.
- 2This product requires antigen retrieval using heat treatment prior to staining of paraffin sections. Tris/EDTA buffer pH 9.0 is recommended for this purpose.
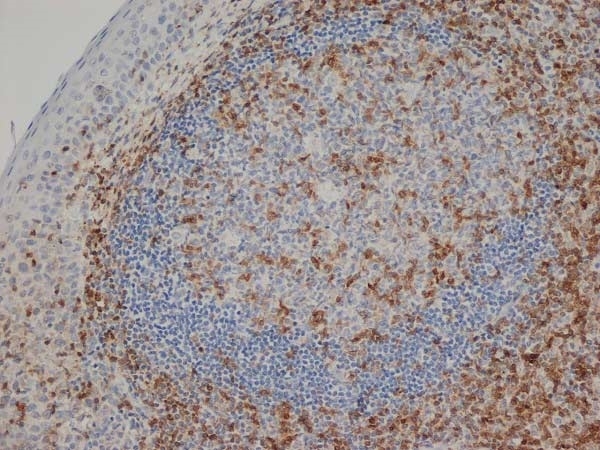
- Histology Positive Control Tissue
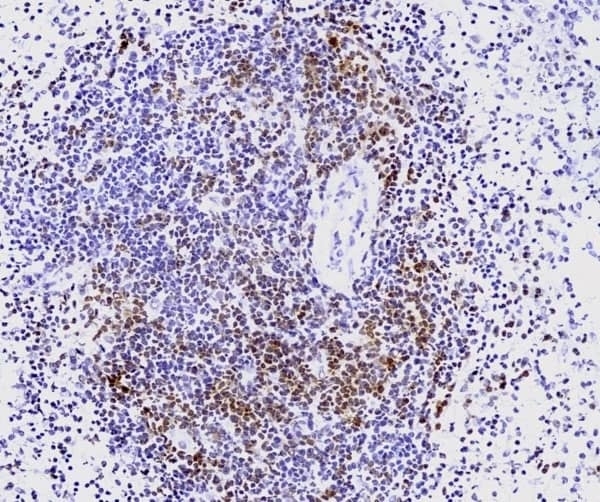
- Human tonsil
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Rat IgG1 Negative Control | MCA6004GA | F | 0.1 mg |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Rat IgG1 Negative Control | ||||||
References for CD3 antibody
-
Jones, M. et al. (1993) Detection of T and B cells in many animal species using cross-reactive anti-peptide antibodies.
J Immunol. 150 (12): 5429-35. -
Cornet, A. et al. (2001) Enterocolitis induced by autoimmune targeting of enteric glial cells: a possible mechanism in Crohn's disease?
Proc Natl Acad Sci U S A. 98: 13306-11. -
Trebst, C. et al. (2003) CC chemokine receptor 8 in the central nervous system is associated with phagocytic macrophages.
Am J Pathol. 162: 427-38. -
Kapturczak, M.H. et al. (2004) Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse.
Am J Pathol. 165 (3): 1045-53. -
Shulga-Morskaya, S. et al. (2004) B cell-activating factor belonging to the TNF family acts through separate receptors to support B cell survival and T cell-independent antibody formation.
J Immunol. 173 (4): 2331-41. -
Pusterla, N. et al. (2006) Multicentric T-cell lymphosarcoma in an alpaca.
Vet J. 171: 181-5. -
Merkler, D. et al. (2006) "Viral déjà vu" elicits organ-specific immune disease independent of reactivity to self.
J Clin Invest. 116: 1254-63. -
Herrmann, I. et al. (2006) Streptococcus pneumoniae Infection aggravates experimental autoimmune encephalomyelitis via Toll-like receptor 2.
Infect Immun. 74: 4841-8.
View The Latest Product References
-
Hudson, K.J. and Bouton, A.H. (2006) Yersinia pseudotuberculosis adhesins regulate tissue-specific colonization and immune cell localization in a mouse model of systemic infection.
Infect Immun. 74: 6487-90. -
Patole, P.S. et al. (2006) Expression and regulation of Toll-like receptors in lupus-like immune complex glomerulonephritis of MRL-Fas(lpr) mice.
Nephrol Dial Transplant 21 (11): 3062-73. -
Beineke, A. et al. (2007) Phenotypical characterization of changes in thymus and spleen associated with lymphoid depletion in free-ranging harbor porpoises (Phocoena phocoena).
Vet Immunol Immunopathol. 117: 254-65. -
Singleton, C.L. et al. (2007) Diagnosis and treatment of chronic T-lymphocytic leukemia in a spotted hyena (Crocuta crocuta).
J Zoo Wildl Med. 38: 488-91. -
Malka, S. et al. (2008) Disseminated lymphoma of presumptive T-cell origin in a great horned owl (Bubo virginianus).
J Avian Med Surg. 22: 226-33. -
Steinberg, J.D. and Keating, J.H. (2008) What is your diagnosis? Cervical mass in a cat.
Vet Clin Pathol. 37: 323-7. -
Tzartos, J.S. et al. (2008) Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis.
Am J Pathol. 172: 146-55. -
Muljono, A. et al. (2009) Primary cutaneous lymphoblastic lymphoma in children: series of eight cases with review of the literature.
Pathology. 41 (3): 223-8. -
Foryst-Ludwig, A. et al. (2010) PPARgamma activation attenuates T-lymphocyte-dependent inflammation of adipose tissue and development of insulin resistance in obese mice.
Cardiovasc Diabetol. 9: 64. -
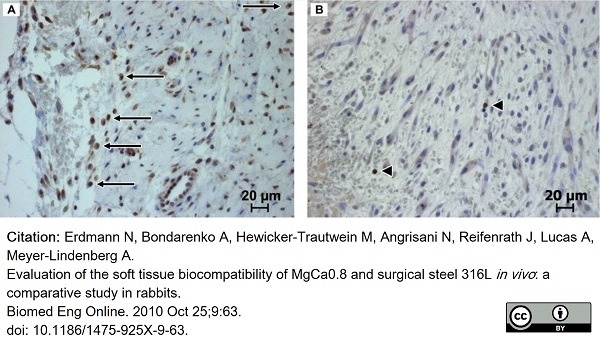
Erdmann, N. et al. (2010) Evaluation of the soft tissue biocompatibility of MgCa0.8 and surgical steel 316L in vivo: a comparative study in rabbits.
Biomed Eng Online. 9: 63. -
Gendronneau, G. et al. (2010) Influence of Hoxa5 on p53 tumorigenic outcome in mice.
Am J Pathol. 176: 995-1005. -
Kleiter, I. et al. (2010) Smad7 in T cells drives T helper 1 responses in multiple sclerosis and experimental autoimmune encephalomyelitis.
Brain. 2010 Apr;133(Pt 4):1067-81. -
Redon, C.E. et al. (2010) Tumors induce complex DNA damage in distant proliferative tissues in vivo.
Proc Natl Acad Sci U S A. 107: 17992-7. -
Colegrove, K.M. et al. (2010) Polyomavirus infection in a free-ranging California sea lion (Zalophus californianus) with intestinal T-cell lymphoma.
J Vet Diagn Invest. 22: 628-32. -
Bartlett SL et al. (2010) Intestinal lymphoma of granular lymphocytes in a fisher (Martes pennanti) and a Eurasian otter (Lutra lutra).
J Zoo Wildl Med. 41 (2): 309-15. -
Dewals B.G., et al. (2011) Malignant catarrhal fever induced by Alcelaphine herpesvirus 1 is characterized by an expansion of activated CD3+CD8+CD4- T cells expressing a cytotoxic phenotype in both lymphoid and non-lymphoid tissues
Vet Res. 42:95 -
Osofsky, A. et al. (2011) T-cell chronic lymphocytic leukemia in a double yellow-headed Amazon parrot (Amazona ochrocephala oratrix).
J Avian Med Surg. 25: 286-94. -
Tosiek, M.J. et al. (2011) CD4+CD25+Foxp3+ regulatory T cells are dispensable for controlling CD8+ T cell-mediated lung inflammation.
J Immunol. 186: 6106-18. -
Osorio, Y. et al. (2011) Identification of small molecule lead compounds for visceral leishmaniasis using a novel ex vivo splenic explant model system.
PLoS Negl Trop Dis. 5:e962. -
Wiessner, C. et al. (2011) The Second-Generation Active A{beta} Immunotherapy CAD106 Reduces Amyloid Accumulation in APP Transgenic Mice While Minimizing Potential Side Effects.
J Neurosci. 31: 9323-31. -
Flatz, L. et al (2011) T cell-dependence of Lassa fever pathogenesis.
PLoS Pathog. 6: e1000836. -
Lau, Q. et al. (2012) Expression and in vitro upregulation of MHCII in koala lymphocytes.
Vet Immunol Immunopathol. 147: 35-43. -
Ruf, M.T. et al. (2012) Chemotherapy-Associated Changes of Histopathological Features of Mycobacterium ulcerans Lesions in a Buruli Ulcer Mouse Model.
Antimicrob Agents Chemother. 56: 687-96. -
Campuzano, O. et al. (2012) Arrhythmogenic right ventricular cardiomyopathy: severe structural alterations are associated with inflammation.
J Clin Pathol. 65 (12): 1077-83. -
Bricker, N.K. et al. (2012) Cytochemical and immunocytochemical characterization of blood cells and immunohistochemical analysis of spleen cells from 2 species of frog, Rana (Aquarana) catesbeiana and Xenopus laevis.
Vet Clin Pathol. 41: 353-61. -
Roy, M. et al. (2012) CXCL1 can be regulated by IL-6 and promotes granulocyte adhesion to brain capillaries during bacterial toxin exposure and encephalomyelitis.
J Neuroinflammation. 9: 18. -
Giannitti, F. et al. (2014) Temporal and geographic clustering of polyomavirus-associated olfactory tumors in 10 free-ranging raccoons (Procyon lotor).
Vet Pathol. 51 (4): 832-45. -
Wen, J. et al. (2015) TNF-like weak inducer of apoptosis promotes blood brain barrier disruption and increases neuronal cell death in MRL/lpr mice.
J Autoimmun. 60: 40-50. -
Ito, D. et al. (2015) A double blinded, placebo-controlled pilot study to examine reduction of CD34 +/CD117 +/CD133 + lymphoma progenitor cells and duration of remission induced by neoadjuvant valspodar in dogs with large B-cell lymphoma.
F1000Res. 4: 42. -
Zhang, M.Z. et al. (2015) Inhibition of cyclooxygenase-2 in hematopoietic cells results in salt-sensitive hypertension.
J Clin Invest. 125 (11): 4281-94. -
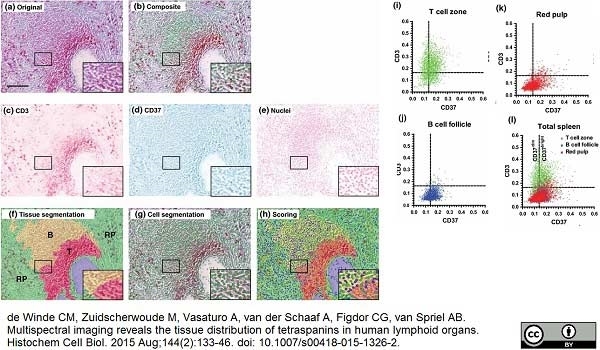
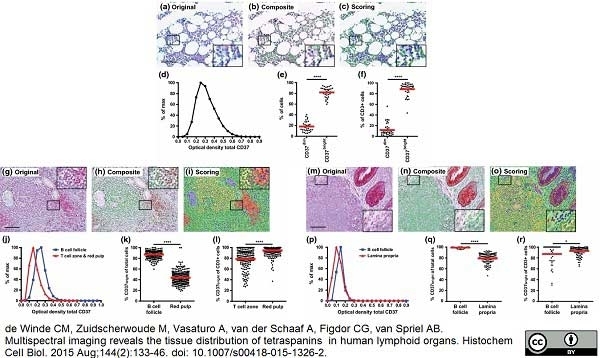
de Winde, C.M. et al. (2015) Multispectral imaging reveals the tissue distribution of tetraspanins in human lymphoid organs.
Histochem Cell Biol. 144 (2): 133-46. -
Velu, V. et al. (2016) Induction of Th1-Biased T Follicular Helper (Tfh) Cells in Lymphoid Tissues during Chronic Simian Immunodeficiency Virus Infection Defines Functionally Distinct Germinal Center Tfh Cells.
J Immunol. 197 (5): 1832-42. -
DaSilva, A.V.A. et al. (2018) Morphophysiological changes in the splenic extracellular matrix of Leishmania infantum-naturally infected dogs is associated with alterations in lymphoid niches and the CD4+ T cell frequency in spleens.
PLoS Negl Trop Dis. 12 (4): e0006445. -
Bonnefont-Rebeix, C. et al. (2016) Characterization of a novel canine T-cell line established from a spontaneously occurring aggressive T-cell lymphoma with large granular cell morphology.
Immunobiology. 221 (1): 12-22. -
Withers, S.S. et al. (2018) Multi-color flow cytometry for evaluating age-related changes in memory lymphocyte subsets in dogs.
Dev Comp Immunol. 87: 64-74. -
Sommer, A. et al. (2016) Infiltrating T lymphocytes reduce myeloid phagocytosis activity in synucleinopathy model.
J Neuroinflammation 13 (1): 174. -
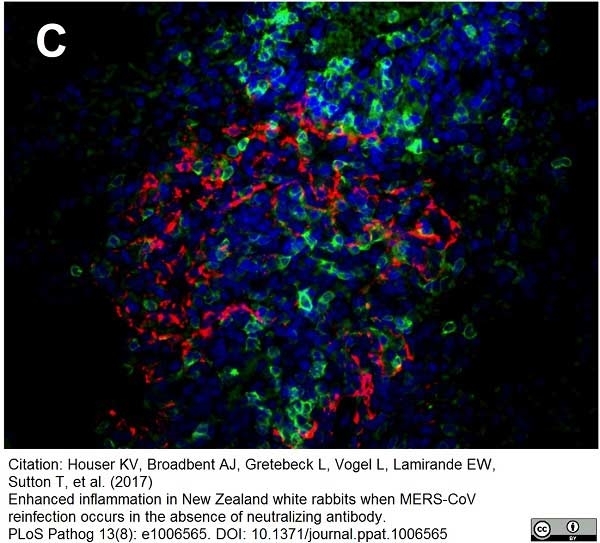
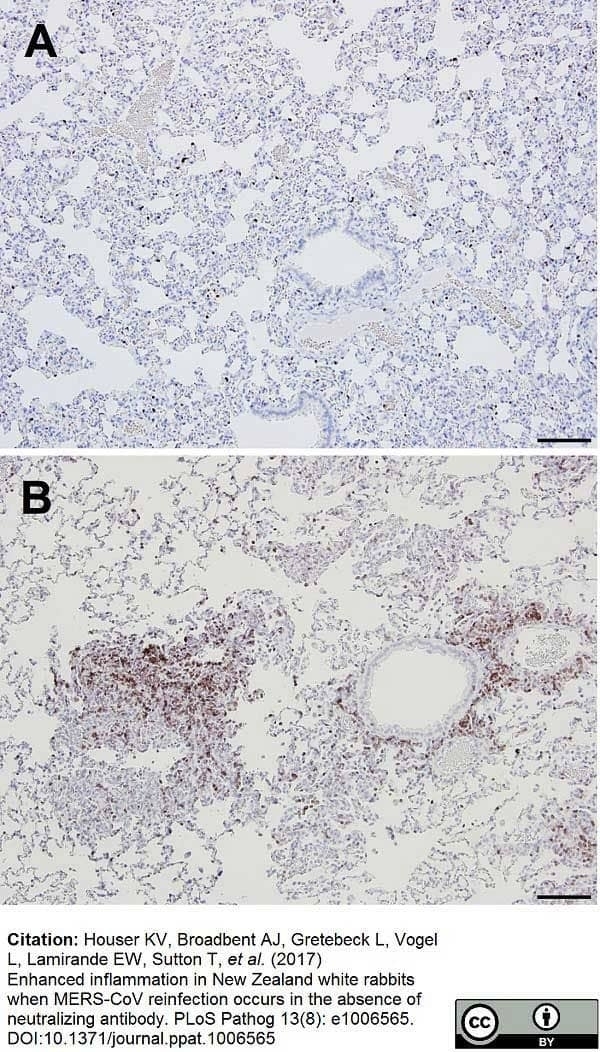
Houser, K.V. et al. (2017) Enhanced inflammation in New Zealand white rabbits when MERS-CoV reinfection occurs in the absence of neutralizing antibody.
PLoS Pathog. 13 (8): e1006565. -
Montes-Cobos, E. et al. (2017) Targeted delivery of glucocorticoids to macrophages in a mouse model of multiple sclerosis using inorganic-organic hybrid nanoparticles.
J Control Release. 245: 157-169. -
Sample, S.J. et al. (2017) Radiographic and magnetic resonance imaging predicts severity of cruciate ligament fiber damage and synovitis in dogs with cranial cruciate ligament rupture.
PLoS One. 12 (6): e0178086. -
Kallikourdis, M. et al. (2017) T cell costimulation blockade blunts pressure overload-induced heart failure.
Nat Commun. 8: 14680. -
Sparger, E.E. et al. (2018) Investigation of immune cell markers in feline oral squamous cell carcinoma.
Vet Immunol Immunopathol. 202: 52-62. -
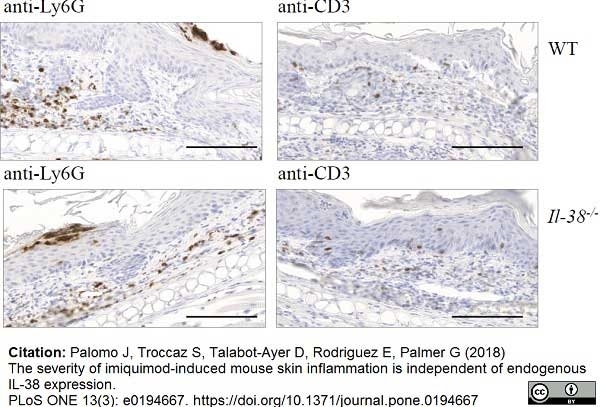
Palomo, J. et al. (2018) The severity of imiquimod-induced mouse skin inflammation is independent of endogenous IL-38 expression.
PLoS One. 13 (3): e0194667. -
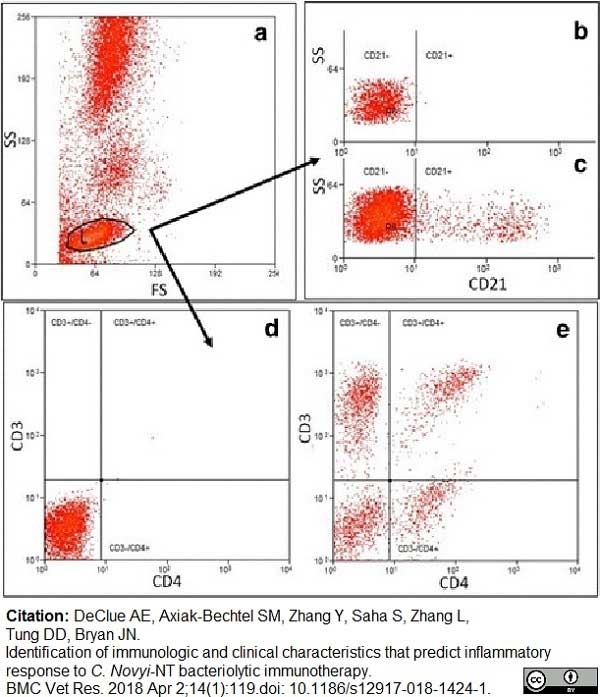
Declue, A.E. et al. (2018) Identification of immunologic and clinical characteristics that predict inflammatory response to C. Novyi-NT bacteriolytic immunotherapy.
BMC Vet Res. 14 (1): 119. -
Pellegrini, S. et al. (2019) Selective local irradiation improves islet engraftment and survival in intra-bone marrow islet transplantation.
Cytotherapy. 21 (10): 1025-32. -
Basu, A. et al. (2019) Association of PD-L1, PD-L2, and Immune Response Markers in Matched Renal Clear Cell Carcinoma Primary and Metastatic Tissue Specimens.
Am J Clin Pathol. 151 (2): 217-25. -
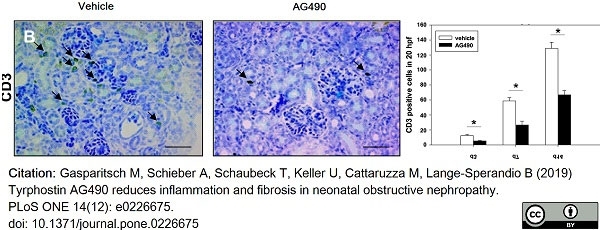
Gasparitsch, M. et al. (2019) Tyrphostin AG490 reduces inflammation and fibrosis in neonatal obstructive nephropathy.
PLoS One. 14 (12): e0226675. -
Thiele, L.S.N. et al. (2020) Functional relevance of the multi-drug transporter abcg2 on teriflunomide therapy in an animal model of multiple sclerosis.
J Neuroinflammation. 17 (1): 9. -
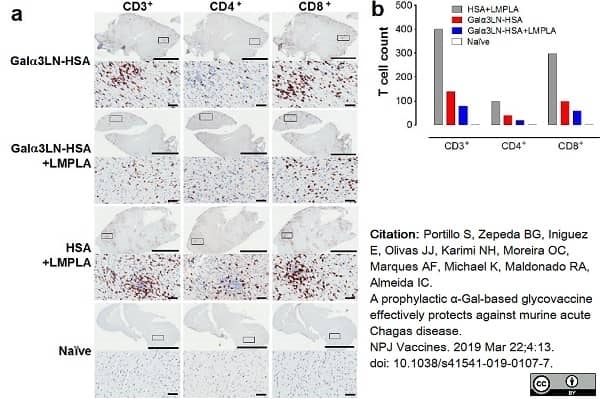
Portillo, S. et al. (2019) A prophylactic α-Gal-based glycovaccine effectively protects against murine acute Chagas disease.
NPJ Vaccines. 4: 13. -
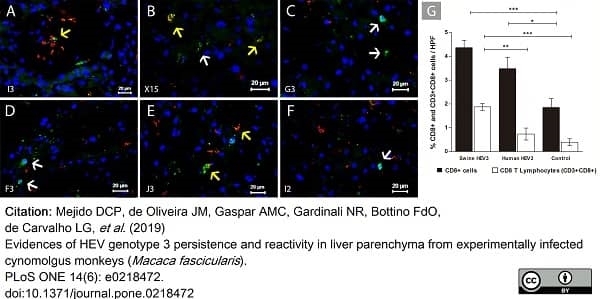
Mejido, D.C.P. et al. (2019) Evidences of HEV genotype 3 persistence and reactivity in liver parenchyma from experimentally infected cynomolgus monkeys (Macaca fascicularis.).
PLoS One. 14 (6): e0218472. -
Choi, S.C. et al. (2020) Gut microbiota dysbiosis and altered tryptophan catabolism contribute to autoimmunity in lupus-susceptible mice.
Sci Transl Med. 12 (551): eaax2220. -
Khodadoust, M.S. et al. (2020) Pembrolizumab in Relapsed and Refractory Mycosis Fungoides and Sézary Syndrome: A Multicenter Phase II Study.
J Clin Oncol. 38 (1): 20-8. -
Ricat, C.M. et al. (2020) Immunohistochemical Findings in Idiopathic Inflammatory Bowel Disease in Nine Cats
BioMed Res Int. 2020: 1-6. -
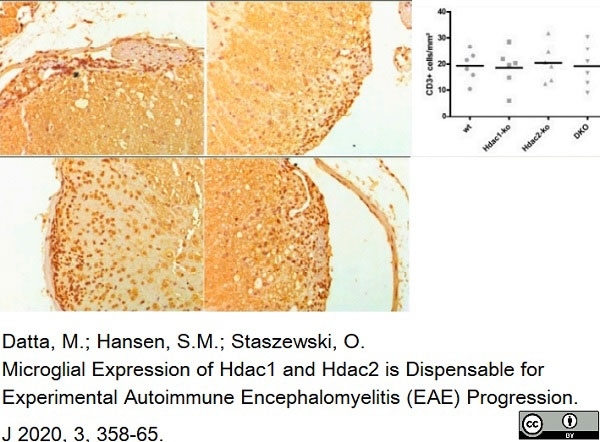
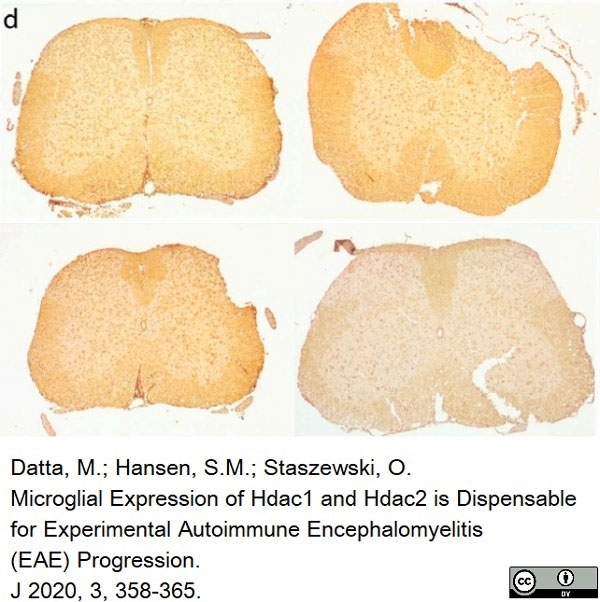
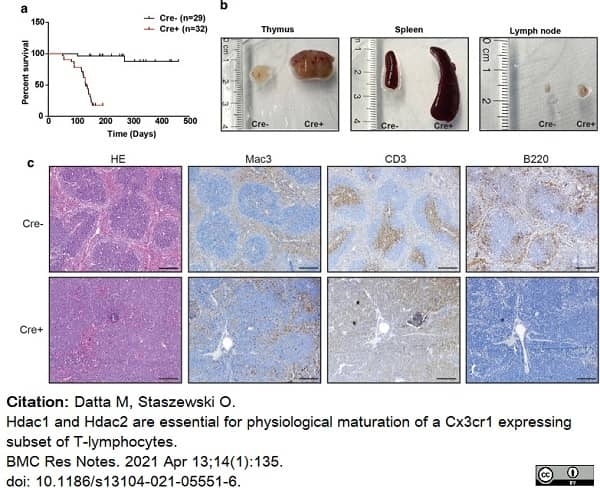
Datta, M. et al. (2020) Microglial Expression of Hdac1 and Hdac2 is Dispensable for Experimental Autoimmune Encephalomyelitis (EAE) Progression
J. 3 (4): 358-65. -
Bagnoud, M. et al. (2020) c-Jun N-Terminal Kinase as a Therapeutic Target in Experimental Autoimmune Encephalomyelitis.
Cells. 9(10): 2154. -
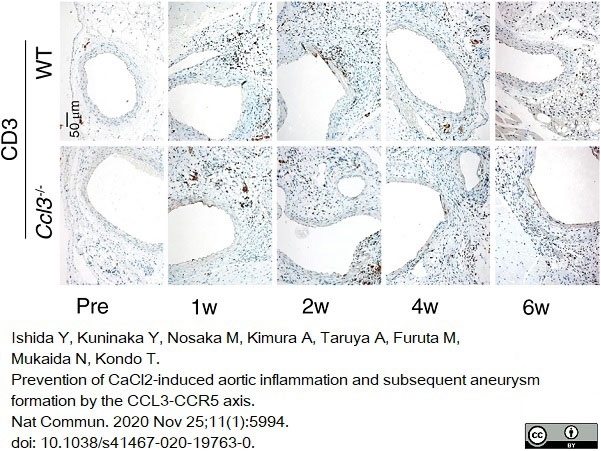
Ishida, Y. et al. (2020) Prevention of CaCl2-induced aortic inflammation and subsequent aneurysm formation by the CCL3-CCR5 axis.
Nat Commun. 11 (1): 5994. -
Nishri, Y. et al. (2020) Continuous Immune-Modulatory Effects of Human Olig2+ Precursor Cells Attenuating a Chronic-Active Model of Multiple Sclerosis.
Mol Neurobiol. 57 (2): 1021-34. -
Ricart, C.M. et al. (2020) Immunohistochemical Findings in Idiopathic Inflammatory Bowel Disease in Nine Cats
BioMed Research International. 2020: 1-6. -
Berghoff, S.A. et al. (2021) Microglia facilitate repair of demyelinated lesions via post-squalene sterol synthesis.
Nat Neurosci. 24 (1): 47-60. -
Phillips, D. et al. (2021) Immune cell topography predicts response to PD-1 blockade in cutaneous T cell lymphoma.
Nat Commun. 12 (1): 6726. -
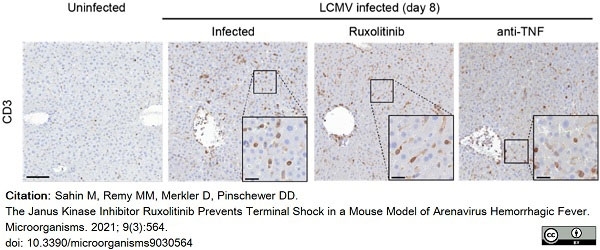
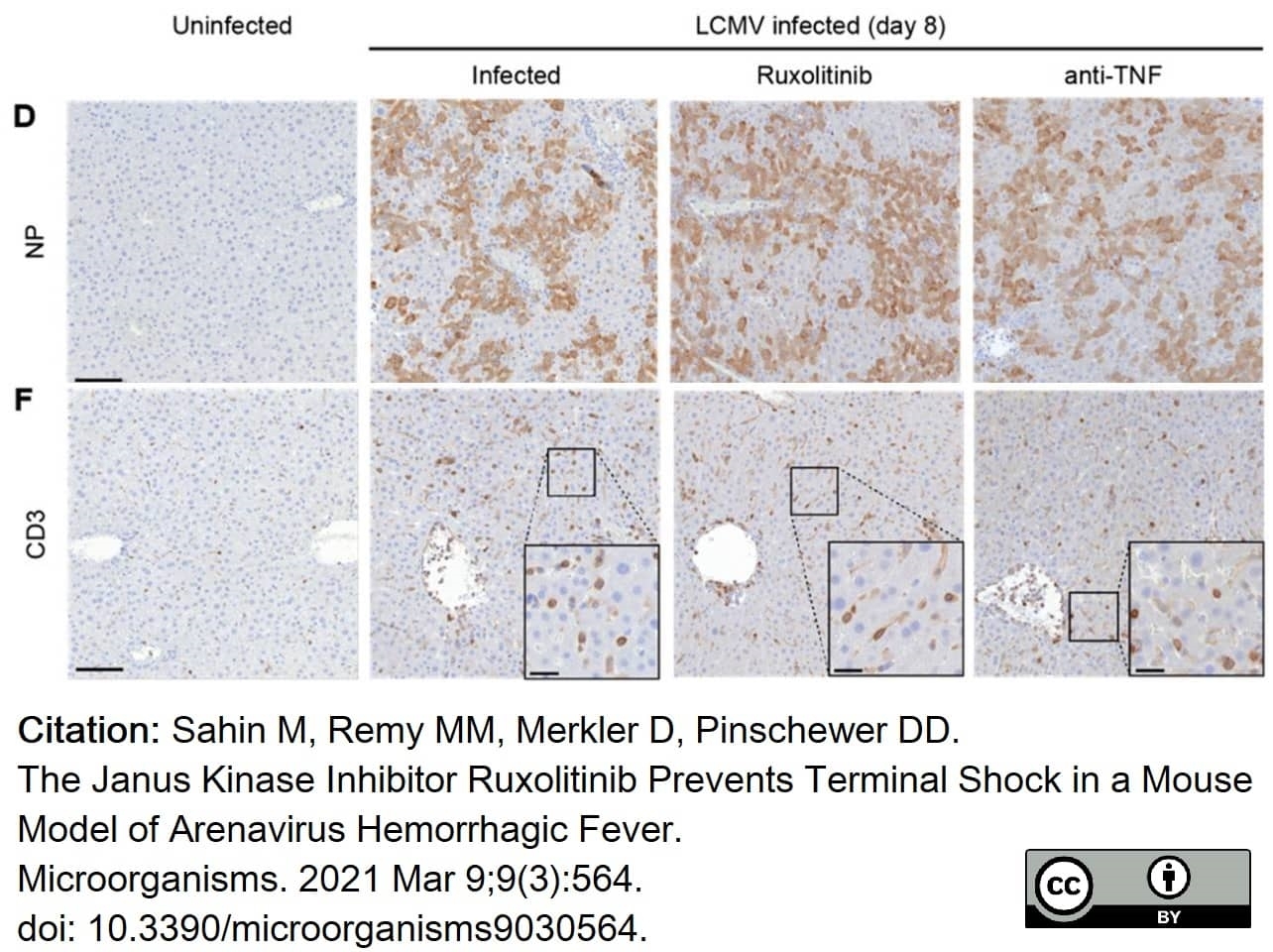
Sahin, M. et al. (2021) The Janus Kinase Inhibitor Ruxolitinib Prevents Terminal Shock in a Mouse Model of Arenavirus Hemorrhagic Fever.
Microorganisms. 9(3):564. -
Jala, V.R. et al. (2021) Absence of CCR2 reduces spontaneous intestinal tumorigenesis in the Apc(Min) (/+) mouse model.
Int J Cancer. Jan 26 [Epub ahead of print]. -
Tigano, M. et al. (2021) In Vivo Analysis of mtDNA Replication at the Single Molecule Level and with High Resolution.
Methods Mol Biol. 2192: 21-34. -
Srivastava, S. et al. (2021) Immunogenic Chemotherapy Enhances Recruitment of CAR-T Cells to Lung Tumors and Improves Antitumor Efficacy when Combined with Checkpoint Blockade.
Cancer Cell. 39 (2): 193-208.e10. -
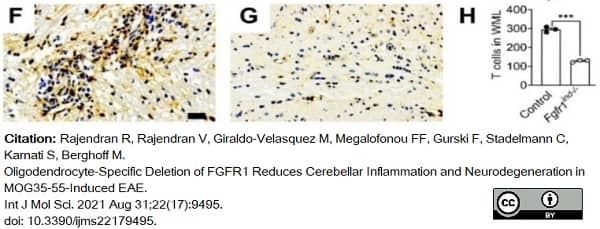
Rajendran, R. et al. (2021) Oligodendrocyte-Specific Deletion of FGFR1 Reduces Cerebellar Inflammation and Neurodegeneration in MOG35-55-Induced EAE.
Int J Mol Sci. 22 (17): 9495. -
Häusler, D. et al. (2021) CNS inflammation after natalizumab therapy for multiple sclerosis: A retrospective histopathological and CSF cohort study.
Brain Pathol. 31 (6): e12969. -
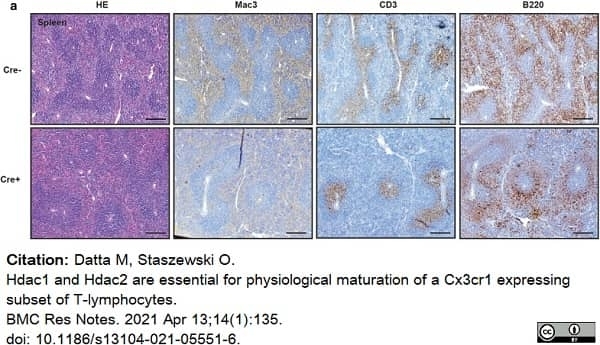
Datta, M. & Staszewski, O. (2021) Hdac1 and Hdac2 are essential for physiological maturation of a Cx3cr1 expressing subset of T-lymphocytes.
BMC Res Notes. 14 (1): 135. -
Sahin, M. et al. (2021) The Janus Kinase Inhibitor Ruxolitinib Prevents Terminal Shock in a Mouse Model of Arenavirus Hemorrhagic Fever
Microorganisms. 9 (3): 564. -
Winkler, A. et al. (2021) Blood-brain barrier resealing in neuromyelitis optica occurs independently of astrocyte regeneration.
J Clin Invest. 131 (5): e141694. -
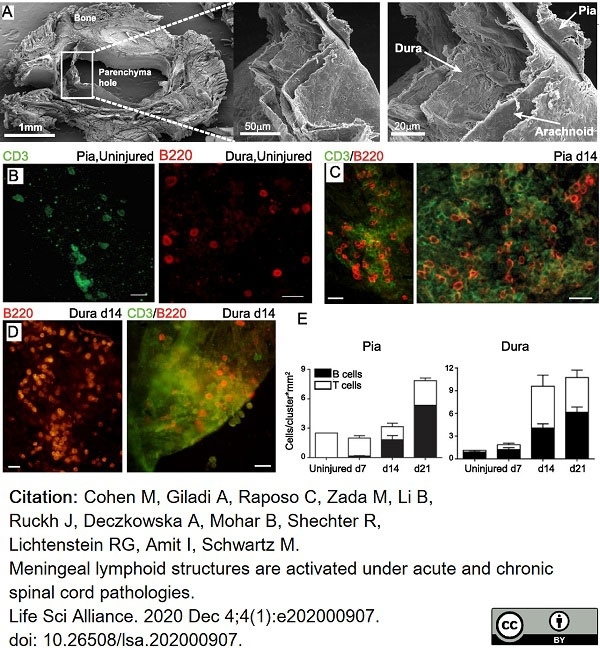
Cohen, M. et al. (2021) Meningeal lymphoid structures are activated under acute and chronic spinal cord pathologies.
Life Sci Alliance. 4 (1): e202000907. -
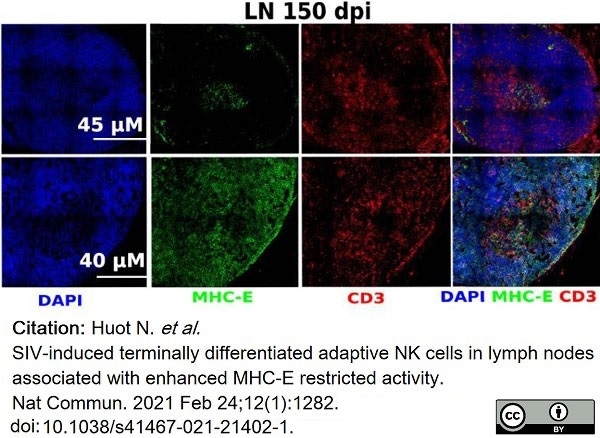
Huot, N. et al. (2021) SIV-induced terminally differentiated adaptive NK cells in lymph nodes associated with enhanced MHC-E restricted activity.
Nat Commun. 12 (1): 1282. -
Arad, T. et al. (2021) CD200 -dependent and -independent immune-modulatory functions of neural stem cells.
Stem Cell Res. 56: 102559. -
Bianchi, A. et al. (2021) Moderate Exercise Inhibits Age-Related Inflammation, Liver Steatosis, Senescence, and Tumorigenesis.
J Immunol. ji2001022. -
Watts, D. et al. (2021) Transient Depletion of Foxp3(+) Regulatory T Cells Selectively Promotes Aggressive β Cell Autoimmunity in Genetically Susceptible DEREG Mice.
Front Immunol. 12: 720133. -
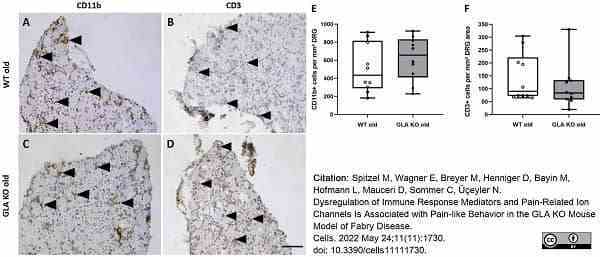
Spitzel, M. et al. (2022) Dysregulation of Immune Response Mediators and Pain-Related Ion Channels Is Associated with Pain-like Behavior in the GLA KO Mouse Model of Fabry Disease.
Cells. 11 (11): 1730. -
Martínez-Vallespín, B. et al. (2022) Evaluation of High Doses of Phytase in a Low-Phosphorus Diet in Comparison to a Phytate-Free Diet on Performance, Apparent Ileal Digestibility of Nutrients, Bone Mineralization, Intestinal Morphology, and Immune Traits in 21-Day-Old Broiler Chickens.
Animals (Basel). 12 (15): 1955. -
Monguió-Tortajada, M. et al. (2022) Acellular cardiac scaffolds enriched with MSC-derived extracellular vesicles limit ventricular remodelling and exert local and systemic immunomodulation in a myocardial infarction porcine model.
Theranostics. 12 (10): 4656-70. -
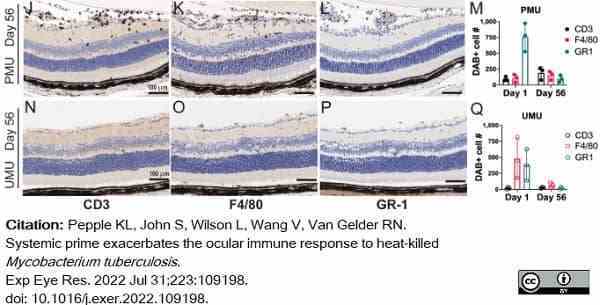
Pepple, K.L. et al. (2022) Systemic prime exacerbates the ocular immune response to heat-killed Mycobacterium tuberculosis..
Exp Eye Res. 223: 109198. -
Cequier, A. et al. (2022) Equine Mesenchymal Stem Cells Influence the Proliferative Response of Lymphocytes: Effect of Inflammation, Differentiation and MHC-Compatibility.
Animals (Basel). 12 (8): 984. -
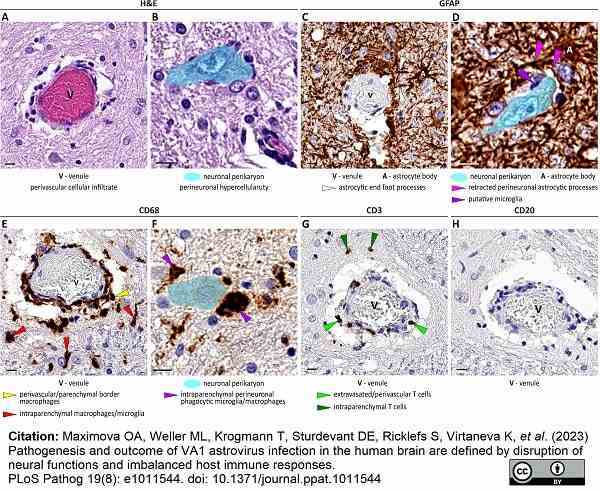
Maximova, O.A. et al. (2023) Pathogenesis and outcome of VA1 astrovirus infection in the human brain are defined by disruption of neural functions and imbalanced host immune responses.
PLoS Pathog. 19 (8): e1011544. -
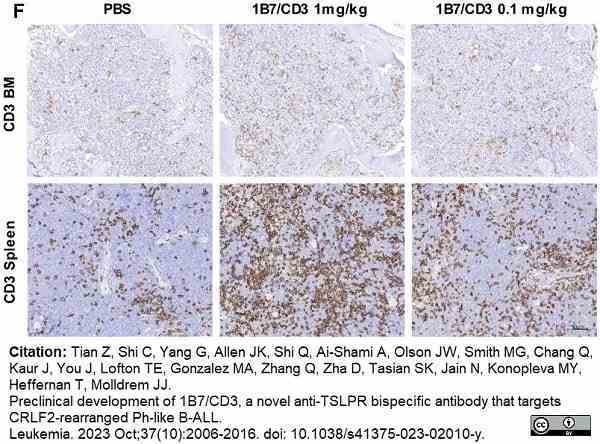
Tian, Z. et al. (2023) Preclinical development of 1B7/CD3, a novel anti-TSLPR bispecific antibody that targets CRLF2-rearranged Ph-like B-ALL.
Leukemia. 37 (10): 2006-16. -
Gogulamudi, V.R. et al. (2023) Heterozygosity for ADP-ribosylation factor 6 suppresses the burden and severity of atherosclerosis.
PLoS One. 18 (5): e0285253. -
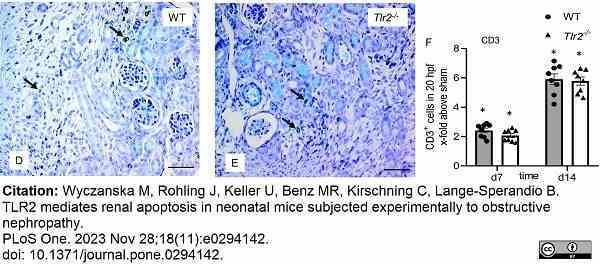
Wyczanska, M. et al. (2023) TLR2 mediates renal apoptosis in neonatal mice subjected experimentally to obstructive nephropathy.
PLoS One. 18 (11): e0294142. -
Enz, L.S. et al. (2023) An Animal Model for Chronic Meningeal Inflammation and Inflammatory Demyelination of the Cerebral Cortex.
Int J Mol Sci. 24 (18):13893. -
Ishida, Y. et al. (2023) Essential Involvement of Neutrophil Elastase in Acute Acetaminophen Hepatotoxicity Using BALB/c Mice.
Int J Mol Sci. 24 (9):7845. -
Migalska, M. et al. (2023) Cross-reactivity of T cell-specific antibodies in the bank vole (Myodes glareolus).
J Immunol Methods. 520: 113524. -
Martini, V. et al. (2018) A retrospective study of flow cytometric characterization of suspected extranodal lymphomas in dogs.
J Vet Diagn Invest. 30 (6): 830-6. -
DeClue, A.E. et al. (2020) Transportation and Routine Veterinary Interventions Alter Immune Function in the Dog.
Top Companion Anim Med. 39: 100408. -
Rütgen, B.C. et al. (2022) Composition of lymphocyte subpopulations in normal and mildly reactive peripheral lymph nodes in cats.
J Feline Med Surg. 24 (2): 77-90. -
Cha, S. et al. (2023) Non-B, Non-T Acute Lymphoblastic Leukemia in a Cat
Journal of Veterinary Clinics. 40 (4): 298-302. -
Yang, L. et al. (2018) Association of the expression of Th cytokines with peripheral CD4 and CD8 lymphocyte subsets after vaccination with FMD vaccine in Holstein young sires.
Res Vet Sci. 119: 79-84. -
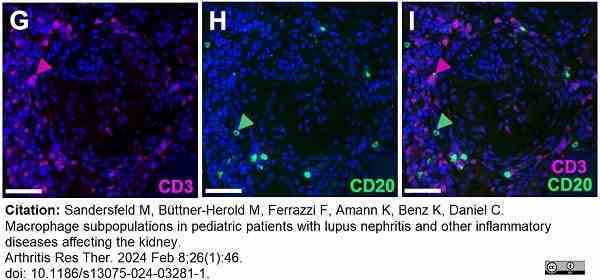
Sandersfeld, M. et al. (2024) Macrophage subpopulations in pediatric patients with lupus nephritis and other inflammatory diseases affecting the kidney.
Arthritis Res Ther. 26 (1): 46. -
Vos, W.G. et al. (2024) T cell specific deletion of Casitas B lineage lymphoma-b reduces atherosclerosis, but increases plaque T cell infiltration and systemic T cell activation
Frontiers in Immunology. 15 04 Mar [Epub ahead of print]. -
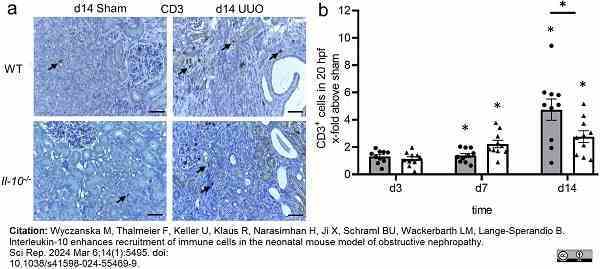
Wyczanska, M. et al. (2024) Interleukin-10 enhances recruitment of immune cells in the neonatal mouse model of obstructive nephropathy.
Sci Rep. 14 (1): 5495.
Further Reading
-
Alterio de Goss, M. et al. (1998) Control of cytomegalovirus in bone marrow transplantation chimeras lacking the prevailing antigen-presenting molecule in recipient tissues rests primarily on recipient-derived CD8 T cells.
J Virol. 72 (10): 7733-44. -
Burudi, E.M. et al. (2002) Regulation of indoleamine 2,3-dioxygenase expression in simian immunodeficiency virus-infected monkey brains.
J Virol. 76 (23): 12233-41. -
Piriou-Guzylack, L. (2008) Membrane markers of the immune cells in swine: an update.
Vet Res. 39: 54.
- RRID
- AB_321245
- UniProt
- P07766
- Entrez Gene
- CD3E
- GO Terms
- GO:0004888 transmembrane receptor activity
- GO:0005057 receptor signaling protein activity
- GO:0005887 integral to plasma membrane
- GO:0007172 signal complex assembly
- GO:0007186 G-protein coupled receptor protein signaling pathway
- GO:0009897 external side of plasma membrane
- GO:0017124 SH3 domain binding
- GO:0019901 protein kinase binding
- GO:0030159 receptor signaling complex scaffold activity
- View More GO Terms
- GO:0031295 T cell costimulation
- GO:0042608 T cell receptor binding
- GO:0046982 protein heterodimerization activity
- GO:0050852 T cell receptor signaling pathway
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Human ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up