Select the Right Antibody for Your Experiments
Overview
Antibodies are invaluable tools for life science research, revealing the presence, quantity, dynamics, and even binding interactions of a target protein. The use of an inappropriate or poor quality antibody can have a significant negative impact on your experiments. Unfortunately, many commercial antibodies have been shown to bind to the wrong target protein, invalidating hard-earned research data. Using an inappropriate antibody, or using an antibody incorrectly, can also lead to the misinterpretation of results. Poor quality antibodies have contributed to a “reproducibility crisis”, with a lack of consistent results observed between research groups (Bordeaux et al. 2010; Prinz et al. 2011; Baker and Dolgin 2017).
Clearly, it is crucial to choose antibodies carefully. With so much information available, how do you know which antibodies are best? To help you, Bio-Rad has developed a list of points to consider when choosing your antibody.
Considerations when choosing an antibody can be simply summarized using “PRECISION”:
P
olyclonal vs monoclonal
R
eproducible data
E
nhanced validation
C
oncentration recommendations
I
mmunogen
S
upport
I
sotype
O
ptimal formulation
N
otable publications
P: Polyclonal vs Monoclonal
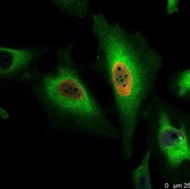
Fig. 1. Proliferating HeLa cells treated with 100 μM BrdU and stained with recombinant PrecisionAb Human Anti-BrdU Antibody (HCA320) at a 1:500 dilution. PureBlu DAPI (1351303) was used as nuclear counterstain.
An antibody can be polyclonal, monoclonal, or recombinant (Figure 1), and this can affect your results:
- A polyclonal antibody is a mixture of antibodies recognizing multiple epitopes on a target protein
- A monoclonal antibody recognizes a single epitope on a target protein
- Recombinant antibodies are made using synthetic approaches rather than animal immunization and can be engineered to bind a single epitope on a target protein
Polyclonal antibodies have a high specificity for detecting low-abundance proteins. They are well-suited to applications that require rapid binding, such as chromatin immunoprecipitation (ChIP). However, they can vary from batch-to-batch and each batch must be validated before use.
Monoclonal antibodies have lower batch-to-batch variability and lower cross-reactivity than polyclonal antibodies, and they are necessary if you need to study a particular epitope. However, they may not have the power to detect low-abundance proteins.
Recombinant antibodies are highly specific and guarantee reproducible results. Their method of production enables long-term security of supply, and does not involve animal immunization.
Bio-Rad’s PrecisionAb Antibodies have been validated in their specific applications, so you can be confident in your results. They may be monoclonal, polyclonal, and/or recombinant, and this information is available on our webpages and datasheets.
R: Reproducible Data
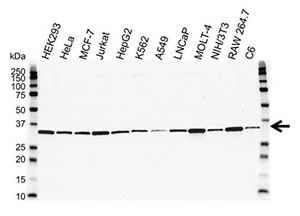
Fig. 2. Western blot analysis of extracts from various cell lines. The experiment was performed using PCNA Antibody (VMA00018), followed by detection with HRP-conjugated Goat Anti-Mouse IgG (1:10,000, STAR207P), and visualized on the ChemiDoc MP Imaging System with 5 sec exposure. Arrow points to PCNA (molecular weight 29 kD).
High quality data on a vendor’s webpage is a good indication that the antibody works appropriately, especially if the data demonstrates the antibody’s performance in your chosen application, such as western blotting or flow cytometry (Figure 2). Transparent image data provide confidence in the antibody, and the figure legends should contain the information required to reproduce the data yourself.
When looking at western blot data, check that the band of interest appears at the correct molecular weight for the target of interest. You can also get an idea of antibody binding and protein abundance by comparing the bands between different cell or tissue lysates. You should check that the western blot is not cropped such that any nonspecific binding is visible and accounted for.
E: Enhanced Validation
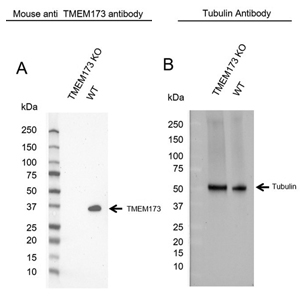
Fig. 3. KO validated TMEM173 (STING) Antibody. Western blot analysis of TMEM173 knockout (KO) HeLa and wild type HeLa (WT) whole cell lysates probed with A, Mouse Anti-TMEM173 Antibody (VMA00456) and B, hFAB Rhodamine Anti-Tubulin Primary Antibody (12004166) as a loading control.
In addition to providing clear data on an antibody, many vendors further validate their antibodies. Enhanced validation is the experimental proof and documentation that a specific antibody is suitable for an intended application or purpose (Weller 2018). The International Working Group on Antibody Validation (IWGAV) defined five validation pillars that provide a framework for validation testing (Uhlen et al. 2016). Validation methods can include genetic strategies such as knockout of the gene producing the protein of interest, or immunoprecipitation followed by mass spectrometry (IP-MS).
At Bio-Rad, we enhance our validation in the PrecisionAb range which is systematically validated by knockout (Figure 3), knockdown, or by IP-MS, and the data is presented on our webpages for transparency.
C: Concentration Recommendations
The vendor should provide a recommended concentration or dilution at which to use the antibody in a specific application, informed by testing. An antibody supplied at 1 mg/ml and recommended for use at 1 µg/ml will give ten tests per vial, but will only give five tests if its recommended use is 2 µg/ml. The PrecisionAb Antibodies range provides data at a concentration that allows at least ten tests from one vial, ensuring value for money.
Always be aware that recommended concentrations are only a guide and should be optimized for your experimental conditions and cell type.
I: Immunogen
The immunogen is the protein, or section of protein, introduced into hosts to generate antibodies. The part of the immunogen bound by the antibody is called the epitope.
For many experiments, it is not necessary to know which region of a protein the antibody binds to, you just need to know that it works. For other experiments, however, knowing the epitope is critical. For example, if you need to detect a region within the C-terminus of a protein, you should look for an antibody that used the C-terminus as an immunogen. This information will appear under “immunogen” on the product webpage or datasheet for PrecisionAb Antibodies.
S: Support
In an ideal world, all experiments would work perfectly first time, but troubleshooting is often needed and technical support from an experienced antibody vendor can be invaluable. It is best to choose a vendor you can rely on when things go wrong. Bio-Rad offers an expert technical support service to help with your experiments and troubleshoot where necessary.
I: Isotype
An antibody consists of two heavy chains and two light chains which combine to form the characteristic “Y” shape. Each heavy chain associates with a light chain to form an antigen-binding site. An antibody can have one of five types of heavy chain, defining its immunoglobulin class (IgG, IgM, IgA, IgE, or IgD), and otherwise known as its “isotype”.
If you have a specific secondary antibody, you should make sure that the isotype of your primary antibody matches the isotype the secondary antibody can detect. The isotype of each PrecisionAb Antibodies is clearly stated, allowing you to accurately plan your experiments.
O: Optimal Formulation
The formulation of an antibody is defined by the buffers, stabilizers, and preservatives present. The ideal formulation will depend on how you plan to use an antibody, so it is worth checking before buying. Most antibodies supplied as a liquid will use either phosphate buffered saline (PBS) or tris-buffered saline (TBS) to maintain a constant pH, note PBS is incompatible with phosphate detection labels. Various preservatives and stabilizers are incompatible with specific applications. For example, antibodies containing sodium azide should not be used for staining live cells as sodium azide is toxic.
N: Notable Publications
In addition to application-specific data, many vendors highlight if an antibody has been used in notable publications and may mention this on their webpages. Published data builds confidence that the antibody works in the “real world” and can therefore be used to generate publication-quality data. However, since not all publications do appear on vendor webpages, we recommend typing the antibody clone name into a publication search engine and looking for papers in which it has been used.
Why Choose PrecisionAb Antibodies?
PrecisionAb Antibodies are supported by high quality, enhanced validation data to build additional confidence in their suitability for your experiments.
The PrecisionAb Antibody range offers a precise solution for reliable data, key features include:
- Evaluation for binding sensitivity and specificity
- Testing on multiple, physiologically relevant cell and tissue lysates expressing endogenous levels of target protein
- Data to support success in a chosen application
- Stringent batch-to-batch quality control
- Verified transfer efficiency using stain-free imaging technology
- Transparent data, blots provided in full and never cropped
All PrecisionAb Antibodies have specific conditions for optimum performance. These include concentration, choice of secondary antibody, dilution, and storage conditions, as well as information on the target lysate and expected experimental outcome. By testing each PrecisionAb Antibody against its own optimized criteria for western blotting, for example, we ensure they perform consistently under specified conditions and generate reproducible results.
In accordance with recommendations by the International Working Group for Antibody Validation (Uhlen et al. 2016), we use various methods to further validate PrecisionAb Antibodies including knockout (KO) and knockdown validation, and immunoprecipitation-mass spectrometry (IPMS) antibody validation.
Learn More on How PrecisionAb Antibodies Are Validated

References
- Baker M and Dolgin E (2017). Cancer reproducibility project releases first results. Nature 541, 269-270.
- Bordeaux J et al. (2010). Antibody validation. Biotechniques 48, 197-209.
- Prinz F et al. (2011). Believe it or not: how much can we rely on published data on potential drug targets? Nat Rev Drug Discov 10, 712.
- Uhlen M et al. (2016). A proposal for validation of antibodies. Nature Methods 13, 823-827.
- Weller MG (2018). Ten basic rules of antibody validation. Anal Chem Insights 13:1177390118757462.