-
US | en

Flow Cytometry Isotype Controls
The use of isotype controls in flow cytometry is controversial and divides researchers (Herzenberg L et al. Maecker HT and Trotter J). You may choose not to use isotype controls but if you do there are some simple rules to follow which will ensure you use them properly and in conjunction with other controls.
What are Isotype Controls?
In flow cytometry, background levels of staining can be a problem especially with rare populations, cells with low expression levels and when building multicolor panels. Isotype controls are antibodies raised against an antigen not found on the cell type or sample analyzed. They have been developed for surface staining and their role is to ensure the observed staining is due to specific antibody binding to the target rather than an artifact. They should not to be used to determine positive versus negative cells or to set gates, and may not be suitable for intracellular staining.
An isotype control will:
- Determine the non-specific binding of an antibody to Fc receptors found on monocytes, macrophages, dendritic and B cells
- Ensure the observed staining is due to specific binding rather than an artifact
- Reveal other non-specific binding of the antibody or fluorophores to cellular components (e.g. RPE and FITC, Takizawa et al. 1993, Hulspas et al. 2009)
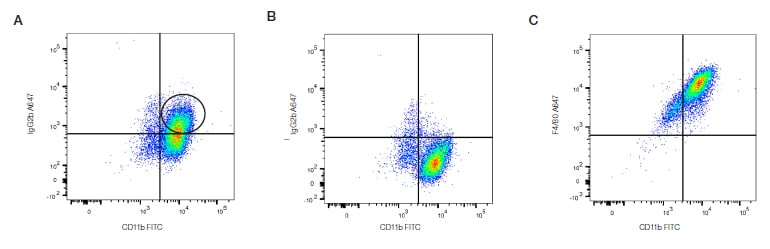
Fig. 20. Isotype controls. J774 macrophages were stained in the absence (A), or in the presence (B and C) of Fc block (BUF041) for CD11b (MCA74F) and F4/80 (MCA497A647), or its isotype, in the presence of 7-AAD (1351102) to exclude dead cells. Without the Fc block there is background staining denoted by the black circle.
The most appropriate isotype control matches:
- The host species
- Ig subclass
- Fluorophore of the primary antibody
If you are using a mouse IgG1 monoclonal antibody that is conjugated to FITC, you should select a mouse IgG1 isotype control conjugated to FITC.
As the fluorophore conjugation to the antibody (known as the F/P ratio) can vary between suppliers, it is best to purchase the isotype from the same supplier as the primary. It is also advisable if possible to use them at the same concentration as the primary antibody.
How to Calculate the F/P ratio of Your Antibodies in Four Easy Steps
To determine the fluorophore : protein ratio you will need to calculate the molar concentrations of both the fluorophore and the protein based on absorbance at known wavelengths in a spectrophotometer and then express this as a ratio. This ratio will be an indication of the average number of dye molecules conjugated to your antibody in your solution.
- Remove any excess fluorophore. Excess fluorophore will interfere with the absorbance measurement.
-
Determine the concentration of protein. Measure the absorbance of the protein at A280 nm. Divide this value by the molar extinction coefficient for your protein.
( e.g. mouse IgG has an extinction coefficient of 203,000 M-1 cm-1). -
Determine the fluorophore concentration. Measure the absorbance of the fluorophore at the wavelength of maximal excitation (e.g. for FITC use 495 nm wavelength). Divide this value by the molar extinction coefficient for the dye.
( e.g. FITC has an extinction coefficient of 75,000 M-1 cm-1). - Determine the labeling. Divide the fluorophore concentration, determined in step 2, by the protein concentration, determined in step 3 to calculate the F/P ratio.
Isotype controls have been optimized for surface staining. Intracellular staining can be affected by binding of both antibody and fluorophore to intracellular components, therefore choice of fluorophore and extra controls may be necessary. Remember isotype controls should not be used to determine compensation levels or the negative population.
Non-specific antibody binding can be reduced by:
- Blocking Fc receptors
- Adding excess protein such as BSA to your buffer
- Titration of your antibody
- Gating out dead cells using live/dead marker
Isoclonic Controls
One alternative to an isotype control is the isoclonic control. This is where cells are stained in the presence of an excess of identical unlabeled antibody. The unlabeled antibody takes up all the binding sites, preventing the labeled antibody from binding specifically. Thus any signal that is detected must come from non-specific binding.
