-
US | en

Apoptosis Flow Cytometry
Apoptosis is a highly regulated process of programmed cell death, involving morphological and biological changes. This process plays an important role in embryogenesis, maintaining an organism’s size, and eliminating damaged or aberrant cells. The importance of apoptosis in human health is underscored by the many diseases resulting from aberrant apoptosis. Dysregulation of apoptosis has been linked to various cancers, neurological and cardiovascular disorders, and autoimmune diseases.
Measuring Apoptosis
One of the most common features of apoptosis that can be measured by flow cytometry is externalization of phosphatidylserine (PS), a phospholipid found in the inner membrane of healthy cells. Annexin V binds to phosphatidyl serine and thus annexin V labeled with fluorophores allow apoptosis to be assessed, usually in combination with a viability dye such as propidium iodide (PI) to distinguish apoptotic from necrotic cells. Healthy cells are negative for both markers, apoptotic cells are positive for annexin V and necrotic cells are positive for both markers. When Jurkat T cells are treated with staurosporine they undergo apoptosis, followed by necrosis. The time course in Figure 33 below shows the increase in annexin V staining after 1 hour as they apoptose, followed by a subsequent increase in necrotic cells by 6 hrs.
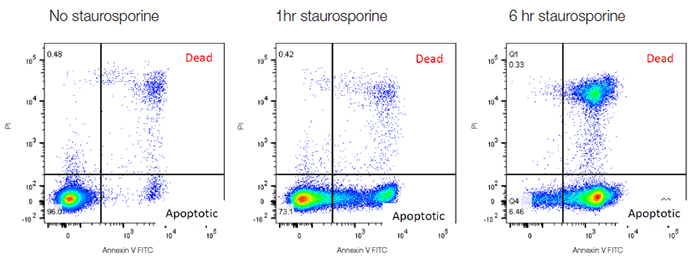
Fig. 33. Annexin V staining to measure apoptosis. Jurkat cells were treated with staurosporine at 1 μM for 0 hr, 1 hr and 6 hr to induce apoptosis. The cells were then stained with annexin V FITC (ANNEX300F) and ReadiDrop™ propidium iodide (1351101). Apoptotic cells positive for annexin V can be seen in the bottom right quadrant and dead cells positive for both annexin and PI in the top right quadrant. Healthy cells are negative for both stains.
Because phosphatidylserine externalization is a dynamic, reversible process until a cell is committed to apoptosis after mitochondrial outer membrane permeabilization (MOMP), annexin V conjugates are unable to distinguish early apoptotis from late apoptosis. Polarity-sensitive indicator of viability and apoptosis (pSIVA) probes are biosensors that reversibly bind to PS and thus turn on and off as PS flips from the outer membrane to the inner membrane. This allows easy comparison of differences in apoptosis rates in response to different experimental treatments in real time.
Alternative Methods
DNA fragmentation, which occurs during the late stages of apoptosis, can also be measured by flow cytometry using the sub-G1 assay. The small, ~180 bp, DNA fragments generated during apoptosis leak out of cells, decreasing the total DNA content of apoptotic cells. By staining DNA with PI, hypodiploid apoptotic cells can be counted in the sub-G1 peak of the PI histogram. Staining with DNA markers will also allow measurement of cell shrinkage in combination with a reduction in the FSC signal.
Early apoptosis can also be measured by potentiomic dyes which assess the reduced mitochondrial potential of cells. Examples of these include tetramethylrhodamine ethylester (TMRE), tetramethylrhodamine methyl ester (TMRM), and JC-1. These lipophilic dyes aggregate in mitochondria of non-apoptotic cells and brightly fluoresce. When the mitochondrial membrane potential collapses the dye disperses into the cytoplasm in its monomeric form leading to reduced fluorescence or a change in color. These dyes can be combined with other apoptosis markers such as fluorophore labeled inhibitor of caspase assays (FLICA) which fluoresce in the presence of caspase and with antibodies against specific caspases.
Resources and Products
We have many resources and products available which have been optimized for flow cytometry and other common antibody applications, find out more about apoptosis and how our apoptosis reagents can help you.
FLICA™ is a trademark of Immunochemistry Technologies, LLC.
pSIVA™ is a trademark of Novus Biologicals and is protected under patent no. 8,541,549.
