Chapter 4: Data Analysis
Overview
The data produced with a Western blot is usually quite easy to interpret. In the majority of cases, bands corresponding to the target protein will become visible upon treatment of the blot with substrate. Their identity is confirmed by comparison to molecular weight markers (for size) and a positive control (size and signal).
In some cases the data may be more complex, showing unexpected sizes, multiple bands, or alteration in bands following a particular treatment. The focus of this chapter is analysis and as such the data itself will be discussed along with examples of different types of Western blot data from research and clinical settings to demonstrate the flexibility of the technique.
If there are no bands on the blot, or if there are unexplained blotches or uneven signal, troubleshooting advice is offered in Chapter 6.
Quantitation
It is very important to be aware that the data produced with a Western blot is typically considered to be semi-quantitative. That is, it provides a relative comparison of protein levels, but not an absolute measure of quantity for a specific target protein in a particular experiment.
The reason for this is two-fold: first, there will be variations in loading and transfer rates between the samples in separate lanes and on separate blots that will need to be normalized before a more precise comparison can be made; second, the signal generated will not be linear across the concentration range of samples due to substrate availability and linear responsiveness of the detection method.
Since the signal produced is not linear, it should not be used to attach a precise concentration to a particular sample. ELISAs are more suitable for this purpose and generally more sensitive.
Normalization
In order to compare target protein expression levels between several different samples on the same blot or across blots, it is necessary to use a loading control to normalize the data. Loading controls are not actually anything that is loaded onto the gel; instead, they refer to a means of equalizing differences in gel loading and transfer rates between samples.
They are not required for every gel that is run, but are necessary for publication quality work, and when the actual signal level between samples is compared. Typically, the blot is probed with an antibody to a well-characterized housekeeping gene which is used as a general measure of protein expression levels in the cells used as a source for a particular sample.
Probing with the housekeeping gene antibody can be carried out along with the target antibody, separately by cutting a blot between the expected band locations, or later after the blot has been stripped of previously bound antibodies. Common loading controls measure the levels of GAPDH, beta actin, tubulin, and histones. These proteins vary in molecular weight and should be carefully chosen depending upon the target protein and experimental conditions since there can be some variation in their signal.
In the Western blot below, Lnk (lymphocyte adaptor protein) expression is compared to non-infected cells and an unrelated protein as a negative control. The matched anti-tubulin probed section of the blot demonstrates that an equivalent amount of sample was loaded in each lane.
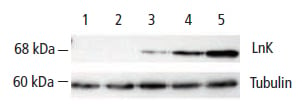
Figure 15: Lnk Expression in Endothelial Cells Following Treatment with TNF a. The Western blot was probed first with anti-Lnk antibody (AHP1003), showing an increase in Lnk expression over time in lanes 3-5. It was then stripped and reprobed with an anti-tubulin antibody to confirm loading equivalence. Lane 1: Non-infected cells. Lane 2: Negative transfection control-AdGFP. Lanes 3-5: 1, 2, and 3 hours post TNF treatment
Densitometers and Analysis Software
In order to make a permanent record, or to get an objective measure of the signal generated on a Western blot, a densitometer is used to scan the blot or film. Imaging software is then used to compare the signal generated by the bands detected on the Western blot.
ECL signal can also be detected with CCD cameras, which usually have a better linear range than film and associated analysis software. Consult the manual for the densitometer or imaging system in use for specific instructions.
| Chapter 3: Test Blots, Slot Blots, and Dot Blots | WB Example: Detecting or Characterizing Protein Expression |





